Degradation of bradykinin by isolated neutral endopeptidases of brain and pituitary.
S Wilk, M Orlowski
Index: Biochem. Biophys. Res. Commun. 90 , 1, (1979)
Full Text: HTML
Abstract
The degradation of bradykinin by a highly purified preparation of rabbit brain prolyl endopeptidase and by an apparently homogeneous preparation of a bovine pituitary cation-sensitive neutral endopeptidase was studied. Peptide fragments were separated and isolated by high performance liquid chromatography and identified by amino acid analysis. Prolyl endopeptidase rapidly cleaves bradykinin at the Pro 7-Phe 8 bond. A slower cleavage also occurs at the Pro 3-Gly 4 bond. Cation-sensitive neutral endopeptidase splits bradykinin at the Phe 5-Ser 6 bond. These enzymes may participate in the regulation of brain concentrations of bradykinin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
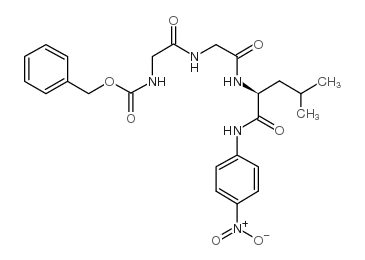 |
Z-Gly-Gly-Leu-pNA
CAS:53046-98-3 |
C24H29N5O7 |
|
A new chromogenic substrate for subtilisin.
1974-12-01 [Anal. Biochem. 62 , 371-376, (1974)] |
|
Evidence that pituitary cation-sensitive neutral endopeptida...
1983-03-01 [J. Neurochem. 40 , 842-849, (1983)] |
|
A synthetic endopeptidase substrate hydrolyzed by the bovine...
1984-05-01 [Exp. Eye Res. 38(5) , 477-83, (1984)] |
|
Activity of Subtilisin Carlsberg in macromolecular crowding.
2007-03-01 [J. Photochem. Photobiol. B, Biol. 86(3) , 199-206, (2007)] |
|
Technical note: quantification of multicatalytic proteinase ...
1993-12-01 [J. Anim. Sci. 71(12) , 3301-6, (1993)] |