| Structure | Name/CAS No. | Articles |
|---|---|---|
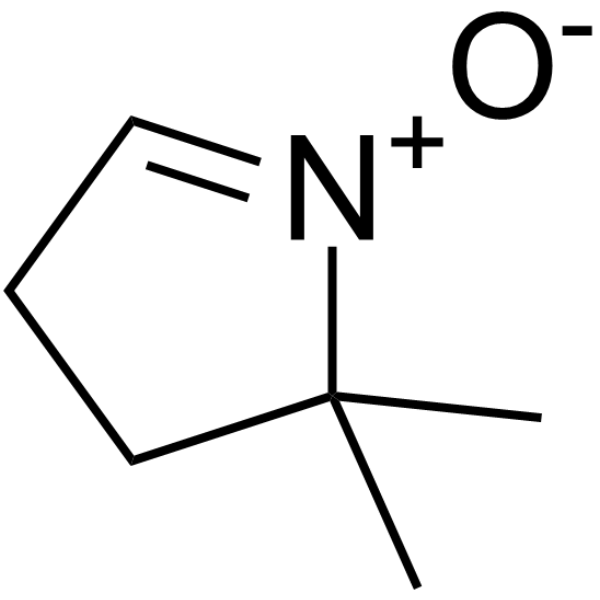 |
DMPO
CAS:3317-61-1 |
|
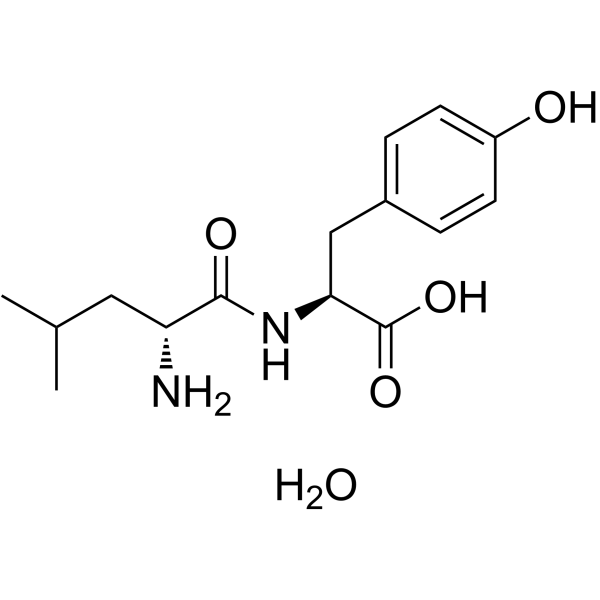 |
H-D-Leu-Tyr-OH
CAS:3303-29-5 |