Effects of radio frequency magnetic fields on iron release from cage proteins.
Oscar Céspedes, Shoogo Ueno
Index: Bioelectromagnetics 30(5) , 336-42, (2009)
Full Text: HTML
Abstract
Ferritin, the iron cage protein, contains a superparamagnetic ferrihydrite nanoparticle formed from the oxidation and absorption of Fe(2+) ions. This nanoparticle increases its internal energy when exposed to alternating magnetic fields due to magnetization lag. The energy is then dissipated to the surrounding proteic cage, affecting its functioning. In this article we show that the rates of iron chelation with ferrozine, an optical marker, are reduced by up to a factor of 3 in proteins previously exposed to radio frequency magnetic fields of 1 MHz and 30 microT for several hours. The effect is non-thermal and depends on the frequency-amplitude product of the magnetic field.(c) 2009 Wiley-Liss, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
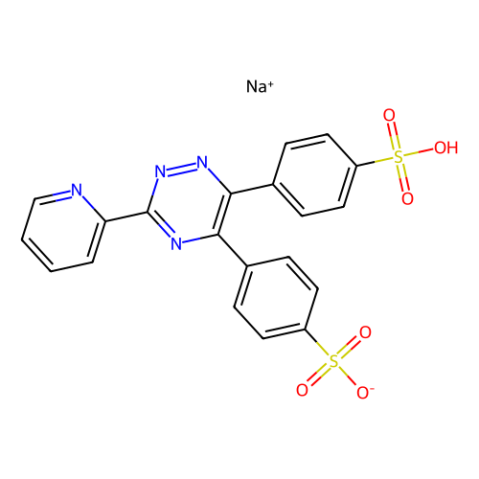 |
ferrozine
CAS:69898-45-9 |
C20H13N4NaO6S2 |
|
[Antioxidant properties of proteins after freezing-thawing].
[Ukr. Biokhim. Zh. 84(1) , 53-9, (2012)] |
|
Characterization of disposable optical sensors for heavy met...
2012-05-30 [Talanta 94 , 123-32, (2012)] |
|
Limitations of the tetramethylmurexide assay for investigati...
2009-07-22 [J. Agric. Food Chem. 57(14) , 6425-31, (2009)] |
|
Antiinflammatory and antioxidant activities of gum mastic.
2010-09-01 [Eur. Rev. Med. Pharmacol. Sci. 14(9) , 765-9, (2010)] |
|
Biological activities of the polysaccharides produced in sub...
2012-01-01 [J. Biomed. Biotechnol. 2012 , 565974, (2012)] |