Quinoline 2-oxidoreductase and 2-oxo-1,2-dihydroquinoline 5,6-dioxygenase from Comamonas testosteroni 63. The first two enzymes in quinoline and 3-methylquinoline degradation.
S Schach, B Tshisuaka, S Fetzner, F Lingens
Index: Eur. J. Biochem. 232(2) , 536-44, (1995)
Full Text: HTML
Abstract
The enzymes catalysing the first two steps of quinoline and 3-methylquinoline degradation by Comamonas testosteroni 63 were investigated. Quinoline 2-oxidoreductase, which catalyses the hydroxylation of (3-methyl-)quinoline to (3-methyl-)2-oxo-1,2-dihydroquinoline, was purified to apparent homogeneity. The native enzyme, with a molecular mass of 360 kDa, is composed of three non-identical subunits (87, 32, and 22 kDa), occurring in a ratio of 1.16:1:0.83. Containing FAD, molybdenum, iron, and acid-labile sulfur in the stoichiometric ratio of 2:2:8:8, the enzyme belongs to the molybdo-iron/sulfur flavoproteins. Molybdopterin cytosine dinucleotide is the organic part of the pterin molybdenum cofactor. Comparison of N-terminal amino acid sequences revealed similarities to a number of procaryotic molybdenum-containing hydroxylases. Especially the N-termini of the beta-subunits of the quinoline 2-oxidoreductases from Comamonas testosteroni 63, Pseudomonas putida 86, and Rhodococcus spec. B1, and of quinoline-4-carboxylic acid 2-oxidoreductase from Agrobacterium spec. 1B showed striking similarities. Further degradation of (3-methyl-)2-oxo-1,2-dihydroquinoline proceeds via dioxygenation at the benzene ring, i.e. at 5,6-position [Schach, S., Schwarz, G., Fetzner, S. & Lingens, F. (1993) Biol. Chem. Hoppe-Seyler 374, 175-181]. 2-Oxo-1,2-dihydroquinoline 5,6-dioxygenase was partially purified; NADH and oxygen are required for the reaction, and the enzymic activity is enhanced 1.5-fold by addition of Fe2+ ions. Unexpectedly, this aromatic ring dioxygenase did not separate into distinct protein components, but is apparently a single-component enzyme. The molecular mass was estimated to be about 260 kDa. 2-Oxo-1,2-dihydroquinoline 5,6-dioxygenase is very thermolabile. However, dithioerythritol and low concentrations of substrate had a moderately stabilizing effect. 2-Oxo-1,2-dihydroquinoline 5,6-dioxygenase is inhibited by sulfhydryl-blocking agents, by metal-chelating agents, and by the flavin analogues quinacrine and acriflavin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
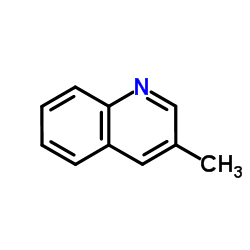 |
3-Methylquinoline
CAS:612-58-8 |
C10H9N |
|
Time-of-flight accurate mass spectrometry identification of ...
2015-08-01 [Anal. Bioanal. Chem 407 , 6159-70, (2015)] |
|
Tetra-μ-benzoato-bis-[(3-methyl-quinoline)copper(II)](Cu-Cu)...
2008-01-01 [Acta Crystallogr. Sect. E Struct. Rep. Online 64(9) , m1141, (2008)] |
|
Microbial metabolism of quinoline and related compounds. XVI...
1993-03-01 [Biol. Chem. Hoppe-Seyler 374(3) , 175-81, (1993)] |
|
Selective synthesis of 2-methylquinoline over zeolites. ...
[Catal. Letters 56(2-3) , 155-58, (1998)] |