Interaction of purified precipitating and non-precipitating (coprecipitating) antibodies with hapten and with haptenated protein. Evidence of an asymmetric antibody molecule.
J R Ronco, E Sciutto, J Leoni, R A Margni, R A Binaghi
Index: Immunology 52(3) , 449-56, (1984)
Full Text: HTML
Abstract
The interaction of monovalent hapten dinitrophenyl epsilon-amino caproic acid (DNP-EACA) with purified IgG1 sheep anti-DNP precipitating and non-precipitating antibodies, and their F(ab')2, F(ab') and Fab fragments, was studied by fluorescence quenching and by a radioimmunoassay. The Scatchard plots of whole non-precipitating antibody and its F(ab')2 fragment showed a bi-modal curve that could be interpreted as due to the existence of two populations of sites with very different affinity for the ligand, each population representing 50% of the total number of sites. The F(ab) fragments of the non-precipitating antibody could be fractionated by immunoadsorption into two populations of high and low affinity whose association constants differed by more than 2 logs. The study of the interaction of whole antibodies with DNP-bovine serum albumin (BSA) demonstrated that each molecule of precipitating antibody can combine with two molecules of antigen but non-precipitating antibody cannot combine with more than one molecule of antigen. It is concluded that the molecule of non-precipitating antibody is asymmetric and has a site of high affinity and another of low affinity. As a consequence of this structure the non-precipitating antibody behaves functionally as univalent and is unable to form precipitates with the multivalent antigen and to activate effector mechanisms.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
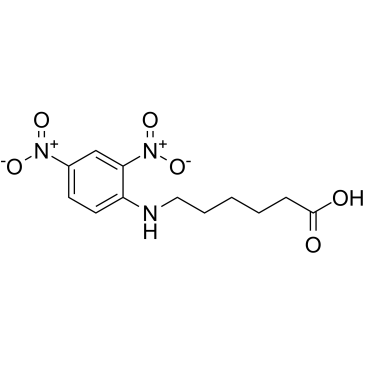 |
N-(2,4-DINITROPHENYL)-6-AMINOHEXANOIC ACID
CAS:10466-72-5 |
C12H15N3O6 |
|
Adaptation of Prostatic-Group-Label Homogeneous Immunoassay ...
1981-09-01 [Clin. Chem. 27(9) , 1499-504, (1981)] |
|
Phospholipase A2 stimulation during cell secretion in rat ba...
1986-01-01 [J. Immunol. 136(1) , 259-63, (1986)] |
|
Multiple resonance fiber-optic sensor with time division mul...
2012-10-01 [Optics Letters 37(19) , 3969-71, (2012)] |
|
Intramolecular heterogeneity of ligand binding by two IgM an...
1983-07-01 [Mol. Immunol. 20(7) , 737-44, (1983)] |