| Structure | Name/CAS No. | Articles |
|---|---|---|
![1-[Decanoyl(methyl)amino]-1-deoxy-D-glucitol Structure](https://image.chemsrc.com/caspic/105/85261-20-7.png) |
1-[Decanoyl(methyl)amino]-1-deoxy-D-glucitol
CAS:85261-20-7 |
|
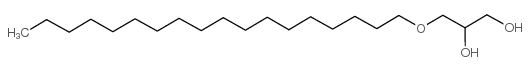 |
4-Oxadocosane-1,2-diol
CAS:544-62-7 |