| Structure | Name/CAS No. | Articles |
|---|---|---|
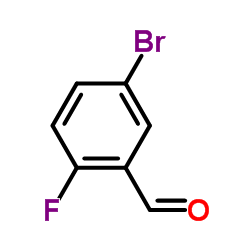 |
5-Bromo-2-fluorobenzaldehyde
CAS:93777-26-5 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
5-Bromo-2-fluorobenzaldehyde
CAS:93777-26-5 |