| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Potassium hexamethyldisilazide
CAS:40949-94-8 |
|
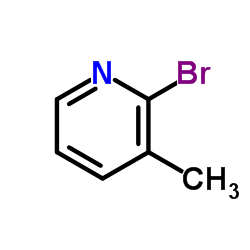 |
2-Bromo-3-methylpyridine
CAS:3430-17-9 |