| Structure | Name/CAS No. | Articles |
|---|---|---|
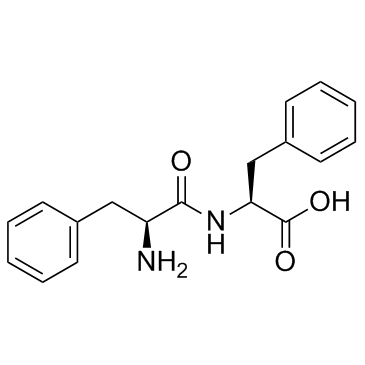 |
H-Phe-Phe-OH
CAS:2577-40-4 |
|
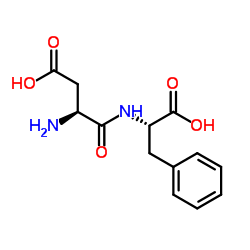 |
L-Aspartyl-L-phenylalanine
CAS:13433-09-5 |
|
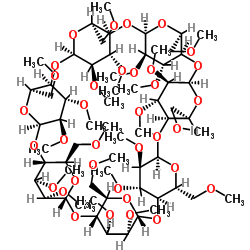 |
Heptakis(2,3,6-tri-O-methyl)-b-cyclodextrin
CAS:55216-11-0 |
|
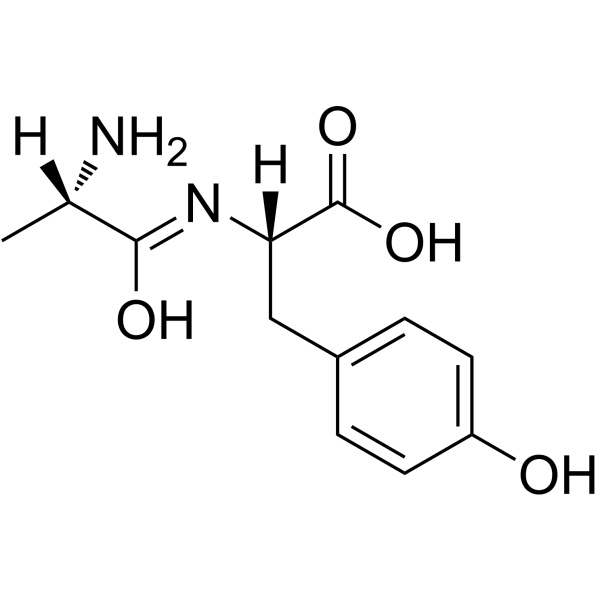 |
H-Ala-Tyr-OH
CAS:3061-88-9 |