NEXAFS spectroscopy of homopolypeptides at all relevant absorption edges: polyisoleucine, polytyrosine, and polyhistidine.
Yan Zubavichus, Andrey Shaporenko, Michael Grunze, Michael Zharnikov
Index: J. Phys. Chem. B 111(33) , 9803-7, (2007)
Full Text: HTML
Abstract
Carefully calibrated high-resolution low-noise near-edge X-ray absorption fine structure spectra of three homopolypetides, viz., polyisoleucine, polytyrosine, and polyhistidine at the C, N, and O K-edges, are compared with the respective spectra of parent amino acids and glycine-derived cyclic dipeptide, 2,5-diketopiperazine. An assignment of the spectral features related to the nitrogen and oxygen atoms constituting the peptide bond is suggested on the basis of a comparative analysis of the experimental spectra as well as theoretical calculations for 2,5-diketopiperazine within the real-space multiple-scattering formalism. A splitting of the pi*-feature in the N K-edge spectra is identified, which is probably sensitive to the dominant conformation type of the peptide molecule (i.e., alpha-helix vs beta-sheet).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
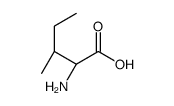 |
POLY-L-ISOLEUCINE
CAS:34464-35-2 |
C6H13NO2 |
|
Direct evidence of the amino acid side chain and backbone co...
2010-02-03 [J. Am. Chem. Soc. 132(4) , 1371-6, (2010)] |
|
Endoplasmic reticulum stress caused by aggregate-prone prote...
2007-11-01 [FEBS J. 274(21) , 5619-27, (2007)] |
|
A solvent model for simulations of peptides in bilayers. I. ...
1999-05-01 [Biophys. J. 76(5) , 2448-59, (1999)] |
|
Molecular theory of the helix-coil transition in polyamino a...
1984-10-01 [Biopolymers 23(10) , 1961-77, (1984)] |
|
Effect of streptomycin on the stoichiometry of GTP hydrolysi...
1985-11-11 [FEBS Lett. 192(1) , 165-9, (1985)] |