| Structure | Name/CAS No. | Articles |
|---|---|---|
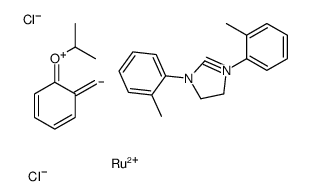 |
Hoveyda-Grubbs Catalyst M721
CAS:927429-61-6 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Hoveyda-Grubbs Catalyst M721
CAS:927429-61-6 |