Biologically active ether lipids. Biotransformation of rac-1(3)-O-alkylglycerols in cell suspension cultures of rape and semisynthesis of 1-O-alkyl-2-palmitoyl-sn-glycero-3-phospho-(N-palmitoyl)ethanolamines, potent antitumor agents.
S S Apte, N Weber, H K Mangold
Index: FEBS Lett. 265(1-2) , 104-6, (1990)
Full Text: HTML
Abstract
Biotransformation of rac-1(3)-O-hexadecylglycerol by photomixotrophic rape (Brassica napus) cells in suspension culture leads to 1-O-hexadecyl-2-acyl-sn-glycero-3-phosphocholines and small proportions of other ether lipids, e.g. 1-O-hexadecyl-2-acyl-sn-glycero-3-phosphoethanolamines. Reaction of the hexadecylacyl-glycerophosphocholines with ethanolamine in the presence of phospholipase D from Streptomyces chromofuscus yields additional hexadecylacylglycerophosphoethanolamines. Partial hydrolysis of the combined hexadecylacylglycerophosphoethanolamines followed by reacylation of the resulting lyso compound with palmitic anhydride gives 1-O-hexadecyl-2-palmitoyl-sn-glycero-3-phospho-(N-palmitoyl) ethanolamine, a nontoxic ether glycerophospholipid with antitumor activity. The corresponding 1-O-tetradecyl,1-O-octadecyl, and 1-O-[(Z)-9'-octadecnyl] derivatives are prepared similarly.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
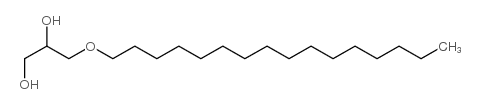 |
1-O-HEXADECYL-RAC-GLYCEROL
CAS:6145-69-3 |
C19H40O3 |
|
Metabolism of fatty acid, glycerol and a monoglyceride analo...
1983-11-01 [Lipids 18(11) , 808-13, (1983)] |
|
Myocardial salvage by 1-O-hexadecyl-Sn-glycerol: possible ro...
1994-09-01 [J. Cardiovasc. Pharmacol. 24(3) , 486-92, (1994)] |
|
Absorption of monoacylglycerols by small intestinal brush bo...
1994-04-19 [Biochemistry 33(15) , 4500-8, (1994)] |
|
Interactions between alkylglycerols and human neutrophil gra...
1990-06-01 [Scand. J. Clin. Lab. Invest. 50(4) , 363-70, (1990)] |
|
Posttranslational regulation of fatty acyl-CoA reductase 1, ...
2010-03-19 [J. Biol. Chem. 285(12) , 8537-42, (2010)] |