Journal of Organic Chemistry
2010-01-15
Asymmetric synthesis of the potent HIV-protease inhibitor, nelfinavir.
Sadagopan Raghavan, V Krishnaiah, B Sridhar
Index: J. Org. Chem. 75(2) , 498-501, (2010)
Full Text: HTML
Abstract
An asymmetric synthesis of nelfinavir is described starting from acrolein and (S)-methyl phenyl sulfoxide. The key features include (a) stereoselective preparation of a beta-protected amino-gamma,delta-unsaturated sulfoxide by the reaction of an alpha-sulfinyl carbanion with an unsaturated t-butyl sulfinylimine, (b) stereoselective bromohydrin formation using the pendant sulfoxide group as an intramolecular nucleophile, and (c) use of commercially or readily prepared inexpensive starting materials.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
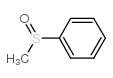 |
methyl phenyl sulfoxide
CAS:1193-82-4 |
C7H8OS |
Related Articles:
More...
|
Murraya koenigii leaf extract inhibits proteasome activity a...
2013-01-01 [BMC Complement Altern. Med. 13 , 7, (2013)] |
|
Resolution of racemic sulfoxides with high productivity and ...
2011-01-01 [Bioresour. Technol. 102(2) , 1537-42, (2011)] |
|
Biocatalytic oxidation by chloroperoxidase from Caldariomyce...
2009-11-21 [Org. Biomol. Chem. 7 , 4604-4610, (2009)] |
|
Synthesis, characterization, reactivity, and catalytic poten...
2006-07-24 [Inorg. Chem. 45(15) , 5924-37, (2006)] |
|
Hydrogen bonding. 32. An analysis of water-octanol and water...
1994-08-01 [J. Pharm. Sci. 83 , 1085, (1994)] |