Solute-solvent interactions and chiral induction in liquid crystals.
Giorgio Celebre, Giuseppina De Luca, Michela Maiorino, Francesca Iemma, Alberta Ferrarini, Silvia Pieraccini, Gian Piero Spada
Index: J. Am. Chem. Soc. 127(33) , 11736-44, (2005)
Full Text: HTML
Abstract
The induction of a cholesteric phase by doping an achiral nematic liquid crystal with an enantiopure solute is a phenomenon that, as in all general supramolecular phenomena of chiral amplification, depends in a subtle way on intermolecular interactions. The micrometric helical deformation of the phase director in the cholesteric phase is generated by the interplay of anisotropy and chirality of probe-medium interactions. In the case of a flexible chiral dopant, the solvent can influence the twisting power in two ways, difficult to disentangle: it is responsible for the solute orientational order, an essential ingredient for the emergence of phase chirality; but also it can affect the dopant conformational distribution and then the chirality of the structures present in the solution. In this work we have investigated methyl phenyl sulfoxide, a flexible, chiral molecule that, when dissolved in different nematics, can produce cholesteric phases of opposite handedness. This peculiar, intriguing sensitivity to the environment makes MPS a suitable probe for a thorough investigation of the effects of solute-solvent interactions on chiral induction in liquid crystals. NMR experiments in various nematic solvents have been performed in addition to twisting power measurements. From the analysis of partially averaged 1H-1H and 13C-1H dipolar couplings, the effects of solvent on solute conformation and orientational order are disentangled, and this information is combined with the modeling of the chirality of intermolecular interactions, within a molecular field theory. The integration of different techniques allows an unprecedented insight into the role of solvent in mediating the chirality transfer from molecule to phase.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
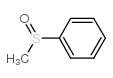 |
methyl phenyl sulfoxide
CAS:1193-82-4 |
C7H8OS |
|
Murraya koenigii leaf extract inhibits proteasome activity a...
2013-01-01 [BMC Complement Altern. Med. 13 , 7, (2013)] |
|
Resolution of racemic sulfoxides with high productivity and ...
2011-01-01 [Bioresour. Technol. 102(2) , 1537-42, (2011)] |
|
Biocatalytic oxidation by chloroperoxidase from Caldariomyce...
2009-11-21 [Org. Biomol. Chem. 7 , 4604-4610, (2009)] |
|
Synthesis, characterization, reactivity, and catalytic poten...
2006-07-24 [Inorg. Chem. 45(15) , 5924-37, (2006)] |
|
Hydrogen bonding. 32. An analysis of water-octanol and water...
1994-08-01 [J. Pharm. Sci. 83 , 1085, (1994)] |