Biochemical characterisation of a hydrophobic ligand binding protein from the tapeworm Hymenolepis diminuta.
N Saghir, P J Conde, P M Brophy, J Barrett
Index: Int. J. Parasitol. 31(7) , 653-60, (2001)
Full Text: HTML
Abstract
The cestode Hymenolepis diminuta contains an abundant, cytoplasmic, hydrophobic ligand, binding protein (H-HLBP). Studies with polarity sensitive probes suggest a single hydrophobic binding site, the results also indicate that the single tryptophan in the molecule (Trp41) is involved in ligand binding. Of the possible physiological ligands tested, only haematin and retinoids (retinol and retinoic acid) show appreciable binding in addition to fatty acids. H-HLBP also binds a range of anthelmintics, again with K(D) values in the nM range. The interaction of anthelmintics with hydrophobic binding proteins may be important in determining drug specificity and site of action and could have a role in the development of drug resistance.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
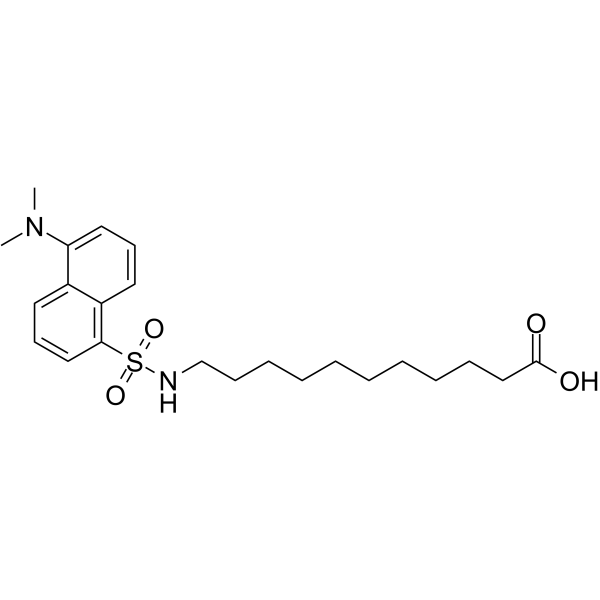 |
DAUDA
CAS:73025-02-2 |
C23H34N2O4S |
|
Lipid-free antigen B subunits from echinococcus granulosus: ...
2015-03-01 [PLoS Negl. Trop. Dis. 9(3) , e0003552, (2015)] |
|
Mutations of recombinant rat liver fatty acid-binding protei...
1996-03-15 [Biochem. J. 314 ( Pt 3) , 943-9, (1996)] |
|
Binding of recombinant rat liver fatty acid-binding protein ...
2015-06-15 [Biochemistry 38(51) , 16932-40, (1999)] |
|
Zn-alpha2-glycoprotein, an MHC class I-related glycoprotein ...
2006-02-21 [Biochemistry 45(7) , 2035-41, (2006)] |
|
The binding of natural and fluorescent lysophospholipids to ...
1995-04-01 [Biochem. J. 307 ( Pt 1) , 305-11, (1995)] |