Synthesis and characterization of fluorinated and iodinated pyrrolopyrimidines as PET/SPECT ligands for the CRF1 receptor.
L Martarello, C D Kilts, T Ely, M J Owens, C B Nemeroff, M Camp, M M Goodman
Index: Nucl. Med. Biol. 28(2) , 187-95, (2001)
Full Text: HTML
Abstract
Fluorine-18 labeled fluorobutyl[2,5-dimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo [2,3-d] pyrimidin-4-yl]ethylamine (FBPPA) and iodine-123 labeled butyl[2,5-dimethyl-7-(4-iodo-2,6-dimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]ethyl-amine (IBPPA) were synthesized in the development of a CRF receptor ligand. The methods of synthesis, in vitro binding assays, radiolabeling and in vivo tissue distribution in rats are described. Fluorine-18 labeled FBPPA was prepared with high specific activity (3 x 10(4) Ci/mmol) by nucleophilic displacement with an average radiochemical yield of 6% (EOB). Iodine-123 labeled IBPPA was prepared by electrophilic iododestannylation with good yield (60%) and high specific activity (3.3 x 10(3) Ci/mmol). The retention of FBPPA and IBPPA in the pituitary was good (1.16% i.d./g and 2.35% i.d./g respectively at 60 min). However, the accumulation of radioactivity in the brain for both radiotracers was very low at all time points of the study, which demonstrated the difficulties for these radiopharmaceuticals to penetrate the blood brain barrier (BBB).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
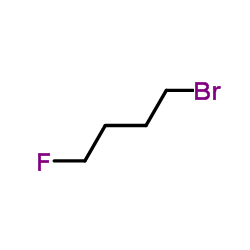 |
1-Bromo-4-fluorobutane
CAS:462-72-6 |
C4H8BrF |
|
Synthesis of 7-halogenated isatin sulfonamides: nonradioacti...
2014-11-26 [J. Med. Chem. 57(22) , 9383-95, (2014)] |
|
Fluorine-substituted cyclofenil derivatives as estrogen rece...
2006-04-20 [J. Med. Chem. 49(8) , 2496-511, (2006)] |
|
Fluorinated Quaternary Ammonium Bromides: Studies on Their T...
[Energy and Fuels 27(9) , 5175-81, (2013)] |
|
Pyrazoles with a “click” 4-[N-(4-fluorobutyl)-1,2,3-t...
[J. Fluor. Chem. 167 , 184-191, (2014)] |