Simultaneous densitometric determination of indomethacin and its degradation products, 4-chlorobenzoic acid and 5-methoxy-2-methyl-3-indoleacetic acid, in pharmaceutical preparations.
J Krzek, M Starek
Index: J. AOAC Int. 84(6) , 1703-7, (2001)
Full Text: HTML
Abstract
A densitometric method was developed for the identification and determination of indomethacin and its degradation products, 4-chlorobenzoic acid and 5-methoxy-2-methyl-3-indoleacetic acid, in pharmaceuticals. To separate these compounds, silica gel-coated thin-layer chromatography plates and the following mobile phase were used: 2-propanol-25% ammonia-water (8 + 1 + 1, v/v). UV densitometric measurements were made by comparing the absorption spectra and Rf values of appropriate standards with the pharmaceutical preparations examined. The conditions for separation were established and a low detection limit was obtained. Average recoveries were 100.69, 90.09, and 91.17% for indomethacin, 4-chlorobeznzoic acid, and 5-methoxy-2-methyl-3-indoleacetic acid, respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
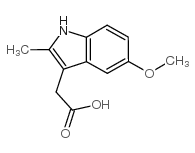 |
5-methoxy-2-methyl-3-indoleacetic acid
CAS:2882-15-7 |
C12H13NO3 |
|
HPLC analysis of indomethacin and its impurities in capsule ...
1982-07-01 [J. Pharm. Sci. 71(7) , 828-30, (1982)] |
|
Accurate assignment of ethanol origin in postmortem urine: l...
2004-06-15 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 805(2) , 223-34, (2004)] |
|
Axonal injury-dependent induction of the peripheral benzodia...
2004-08-01 [Eur. J. Neurosci. 20(3) , 671-83, (2004)] |
|
Expression of mitochondrial benzodiazepine receptor and its ...
1994-09-01 [Cell Growth Differ. 5(9) , 1005-14, (1994)] |
|
High-performance liquid chromatographic method for the deter...
[J. Chromatogr. A. 306 , 315-21, (1984)] |