| Structure | Name/CAS No. | Articles |
|---|---|---|
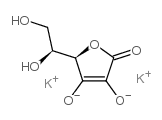 |
L-Ascorbic acid 2-sulfate dipotassium salt
CAS:52174-99-9 |
|
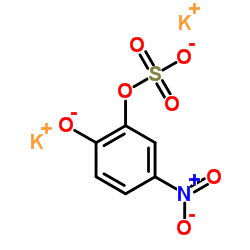 |
Dipotassium 5-nitro-2-oxidophenyl sulfate
CAS:14528-64-4 |