Synthesis, structure and catechol-oxidase activity of copper(II) complexes of 17-hydroxy-16-(N-3-oxo-prop-1-enyl)amino steroids.
Rainer Wegner, Manuela Dubs, Helmar Görls, Christian Robl, Bruno Schönecker, Ernst-G Jäger
Index: Steroids 67(10) , 835-49, (2002)
Full Text: HTML
Abstract
Copper is next to iron the most important element in the biological transport, storage and in redox reactions of dioxygen. A bioanalogous activation of dioxygen with copper complexes is used for catalytical epoxidation, allylic hydroxylation and oxidative coupling of aromatic substrates, for example. With stereochemical information in form of chiral ligands, enantioselective reactions may be possible. Another aspect of interest on copper catalyzed reactions with dioxygen is that the exact mechanism and biological function of some enzymes (especially catechol oxidase) is yet not fully clear. For studies mimicking the copper-containing catechol oxidase appropriate chiral steroid ligands with defined stereochemistry and conformation have been synthesized. The four diastereomeric 16,17-aminoalcohols of the 3-methoxy-estra-1,3,5(10)-triene series have been condensed with salicylic aldehyde and different beta-ketoenols to the chiral ligand types 1-5. These compounds with different steric and electronic properties and different arrangements of the neighboring hydroxy and nitrogen functions were reacted with copper(II) acetate to copper complexes. The structure of these complexes will be discussed. The bioanalogous oxidation of 3,5-di-tbutyl-catechol (dtbc) to the corresponding quinone was catalyzed by most of the complexes, indicating their ability to activate dioxygen. The trans configurations c and d showed an activity one magnitude higher than the cis configurations a and b. Comparing compounds with the same diastereomeric configuration, the main influence was that of the peripheral R(1-3) substituents at the beta-ketoenaminic group which are useful for the fine-tuning of the properties of the copper atoms like redox potential and Lewis acidity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
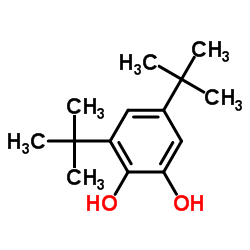 |
3,5-Di-t-butylcatechol
CAS:1020-31-1 |
C14H22O2 |
|
Catechol oxidase activity of a series of new dinuclear coppe...
2008-08-18 [Inorg. Chem. 47(16) , 7083-93, (2008)] |
|
3,5-Di-t-butyl catechol is a potent human ryanodine receptor...
2012-07-01 [Pharmacol. Res. 66(1) , 80-7, (2012)] |
|
A novel tripodal ligand containing three different N-heteroc...
2009-01-01 [Chemistry 15(22) , 5567-76, (2009)] |
|
Chemistry of singlet oxygen--48. Isolation and structure of ...
1987-09-01 [Photochem. Photobiol. 46(3) , 325-30, (1987)] |
|
3,5-di-t-butylcatechol (DTCAT) as an activator of rat skelet...
2005-02-01 [Biochem. Pharmacol. 69(3) , 485-91, (2005)] |