Synthesis of a cleavable protein-crosslinking reagent for the investigation of ribosome structure.
H Peretz, D Elson
Index: Eur. J. Biochem. 63(1) , 77-82, (1976)
Full Text: HTML
Abstract
This communication describes a simple method for synthesizing cleavable bifunctional imido esters of different chain lengths. These reagents, which form covalent crosslinks between lysine residues of proteins, contain a disulfide bond which is cleaved under mild conditions by reducing agents such as 2-mercaptoethanol. The reagents are synthesized via the dithiobisnitrile which is prepared in high yield by reacting the appropriate omega-activated nitrile with sodium polysulfide and is then converted quantitatively to the diimidate. Three such reagents were prepared: dimethyl 3.3'-dithiobispropionimidate, dimethyl 4,4'-dithiobisbutyrimidate, and dimethyl 6-6'-dithiobiscaproimidate. The first was synthesized from acrylonitrile, and the others from the appropriate omega-bromonitriles. Experiments with the bispropionimidate and a test protein, pancreatic ribonuclease, have shown the reagent to be effective in producing multimeric crosslinked complexes, from which monomeric proteins can recovered after treatment with 2-mercaptoethanol. The reagents are suitable for studies of ribosomal structure.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
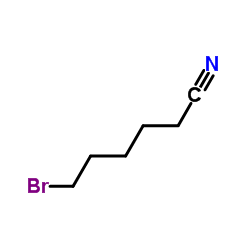 |
6-Bromohexanenitrile
CAS:6621-59-6 |
C6H10BrN |
|
NICKEL-CATALYZED ENANTIOSELECTIVE NEGISHI CROSS-COUPLINGS OF...
2011-05-01 [Organic Synth. 87 , 330, (2010)] |
|
Fluorescent derivatives of σ receptor ligand 1-cyclohexyl-4-...
2011-08-25 [J. Med. Chem. 54 , 5858-67, (2011)] |
|
“Green Chemical" Methods for the Regioselective Synthesis of...
[Latv. Kim. Z. 49(1) , 278-282, (2010)] |
|
Friedel-Crafts alkylation of benzene by normal ?-chloroalkan...
[Bull. Acad. Sci. USSR, Chemistry Series 36(2) , 327-330, (1987)] |