The comparative metabolism of 2,6-dichlorothiobenzamide (Prefix) and 2,6-dichlorobenzonitrile in the dog and rat.
M H Griffiths, J A Moss, J A Rose, D E Hathway
Index: Biochem. J. 98(3) , 770-81, (1966)
Full Text: HTML
Abstract
1. A single oral dose of either [(14)C]Prefix or 2,6-dichlorobenzo[(14)C]nitrile to rats is almost entirely eliminated in 4 days: 84.8-100.5% of (14)C from [(14)C]Prefix is excreted, 67.3-79.7% in the urine, and 85.8-97.2% of (14)C from 2,6-dichlorobenzo-[(14)C]nitrile is excreted, 72.3-80.7% in the urine. Only 0.37+/-0.03% of the dose of [(14)C]Prefix and 0.25+/-0.03% of the dose of 2,6-dichlorobenzo[(14)C]nitrile are present in the carcass plus viscera after removal of the gut. Rats do not show sex differences in the pattern of elimination of the respective metabolites of the two herbicides. The rates of elimination of (14)C from the two compounds in the 24hr. and 48hr. urines are not significantly different (P >0.05) from one another. 2. After oral administration to dogs, 85.9-106.1% of (14)C from [(14)C]Prefix is excreted, 66.6-80.9% in the urine, and 86.8-92.5% of (14)C from 2,6-dichlorobenzo[(14)C]nitrile is excreted, 60.0-70.1% in the urine. Dogs do not show sex differences in the pattern of eliminating the metabolites of either Prefix or 2,6-dichlorobenzonitrile. 3. Dogs and rats do not show species differences in the patterns of elimination of the two herbicides. 4. Prefix and 2,6-dichlorobenzonitrile are completely metabolized; unchanged Prefix and 2,6-dichlorobenzonitrile are absent from the urine and faeces, and from the carcasses when elimination is complete. In the hydrolysed urine of rats dosed with either [(14)C]Prefix or 2,6-dichlorobenzo[(14)C]nitrile, 2,6-dichloro-3-hydroxybenzonitrile accounts for approx. 42% of the (14)C, a further 10-11% is accounted for by 2,6-dichlorobenzamide, 2,6-dichlorobenzoic acid, 2,6-dichloro-3- and -4-hydroxybenzoic acid and 2,6-dichloro-4-hydroxybenzonitrile collectively, and 25-30% by six polar constituents, of which two are sulphur-containing amino acids. 5. In the unhydrolysed urines of rats dosed with either [(14)C]Prefix or 2,6-dichlorobenzo[(14)C]nitrile, there are present free 2,6-dichloro-3- and -4-hydroxybenzonitrile, their glucuronide conjugates, ester glucuronides of the principal aromatic acids that are present in the hydrolysed urines, and two sulphur-containing metabolites analogous to mercapturic acids or premercapturic acids. 6. Prefix is thus extensively transformed into 2,6-dichlorobenzonitrile: R.CS.NH(2)-->R.CN+H(2)S, where R=C(6)H(3)Cl(2). However, the competitive reaction: R.CS.NH(2)+H(2)O-->R.CO.NH(2)+H(2)S takes place to a very limited extent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
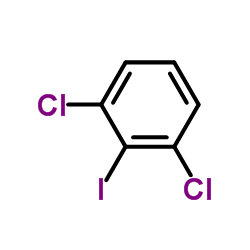 |
1,3-Dichloro-2-iodobenzene
CAS:19230-28-5 |
C6H3Cl2I |
|
Encapsulation of the uranyl dication. Beer S, et al.
[Chem. Sci. 1(1) , 43-47, (2010)] |
|
Non-interconvertible conformers of cupped oxacyclophanes. Gr...
[Tetrahedron Lett. 31(30) , 4271-4274, (1990)] |
|
Synthesis of cuppedophanes and cappedophanes. Two new classe...
[J. Org. Chem. 55(3) , 881-890, (1990)] |
|
Oxacyclophanes based on a m-terphenyl framework. Grew...
[J. Org. Chem. 57(9) , 2721-2726, (1992)] |
|
Zirconium complexes incorporating diaryldiamidoferrocene lig...
[Inorganica Chim. Acta 345 , 216-220, (2003)] |