Identification and functional characterization of a new CYP2C9 variant (CYP2C9*5) expressed among African Americans.
L J Dickmann, A E Rettie, M B Kneller, R B Kim, A J Wood, C M Stein, G R Wilkinson, U I Schwarz
Index: Mol. Pharmacol. 60(2) , 382-7, (2001)
Full Text: HTML
Abstract
CYP2C9 is a polymorphic gene for which there are four known allelic variants; CYP2C9*1, CYP2C9*2, CYP2C9*3, and CYP2C9*4. In the present study, DNA from 140 European Americans and 120 African Americans was examined by single-strand conformational polymorphism and restriction fragment length polymorphism analyses, resulting in the identification of a new CYP2C9 variant, CYP2C9*5. This variant is derived from a C1080G transversion in exon 7 of CYP2C9 that leads to an Asp360Glu substitution in the encoded protein. The CYP2C9*5 variant was found to be expressed only in African Americans, such that approximately 3% of this population carries the CYP2C9*5 allele. The variant was expressed in, and purified from, insect cells infected with a recombinant baculovirus. Comparative kinetic studies using the purified wild-type protein CYP2C9*1; the Ile359Leu variant, CYP2C9*3; and the Asp360Glu variant, CYP2C9*5 were carried out using (S)-warfarin, diclofenac, and lauric acid as substrates. The major effect of the Asp360Glu mutation was to increase the K(m) value relative to that of CYP2C9*1 for all three substrates: 12-fold higher for (S)-warfarin 7-hydroxylation, 5-fold higher for the 4'-hydroxylation of diclofenac, and 3-fold higher for the omega-1 hydroxylation of lauric acid. V(max) values differed less than K(m) values between the CYP2C9*1 and CYP2C9*5 proteins. In vitro intrinsic clearances for CYP2C9*5, calculated as the ratio of V(max)/K(m), ranged from 8 to 18% of CYP2C9*1 values. The corresponding ratio for CYP2C9*3 was 4 to 13%. Accordingly, the in vitro data suggest that carriers of the CYP2C9*5 allele would eliminate CYP2C9 substrates at slower rates relative to persons expressing the wild-type protein.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
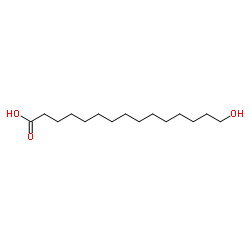 |
15-Hydroxypentadecanoic acid
CAS:4617-33-8 |
C15H30O3 |
|
Profiling of secondary metabolites in root exudates of Arabi...
2014-12-01 [Phytochemistry 108 , 35-46, (2014)] |
|
Matrix-assisted laser desorption/ionization mass spectrometr...
2014-12-01 [Plant J. 80(5) , 926-35, (2014)] |
|
Synthesis and anticancer activities of fatty acid analogs of...
2004-02-01 [Lipids 39(2) , 167-72, (2004)] |
|
Identification of the peroxisomal beta-oxidation enzymes inv...
2004-06-01 [J. Lipid Res. 45(6) , 1104-11, (2004)] |
|
Preparation of 15-hydroxypentadecanoic acid by means of cond...
[Can. J. Chem. 46(23) , 3767-3770, (1968)] |