Organic & Biomolecular Chemistry
2012-09-14
Enantio- and diastereocontrolled conversion of chiral epoxides to trans-cyclopropane carboxylates: application to the synthesis of cascarillic acid, grenadamide and L-(-)-CCG-II.
Pradeep Kumar, Abhishek Dubey, Anand Harbindu
Index: Org. Biomol. Chem. 10(34) , 6987-94, (2012)
Full Text: HTML
Abstract
An efficient high yielding improved method for the enantio- and diastereoselective cyclopropanation of chiral epoxides using triethylphosphonoacetate and base (Wadsworth-Emmons cyclopropanation) is reported. The utility of this protocol is illustrated by concise and practical synthesis of cascarillic acid, grenadamide and L-(-)-CCG-II, a cyclopropane containing natural products.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
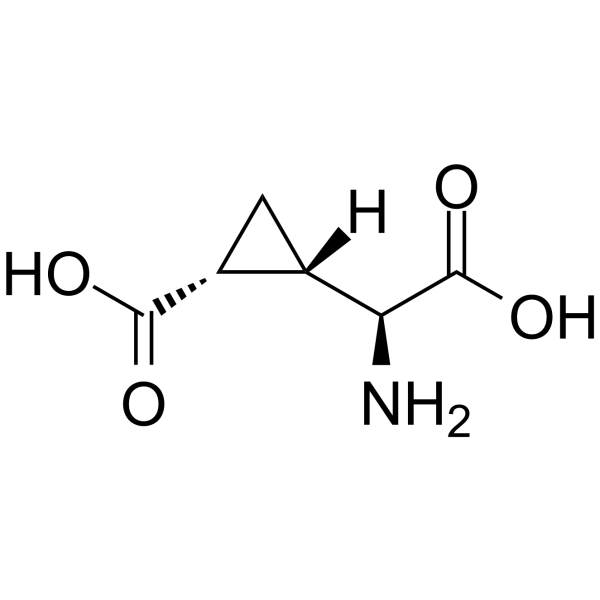 |
L-CCG-IV
CAS:117857-95-1 |
C6H9NO4 |
Related Articles:
More...
|
Binding and transport of [3H](2S,4R)- 4-methylglutamate, a n...
2004-03-15 [J. Neurosci. Res. 75(6) , 751-9, (2004)] |
|
Synthesis, molecular modeling studies, and preliminary pharm...
2007-09-20 [J. Med. Chem. 50 , 4630-41, (2007)] |
|
Involvement of Group II mGluRs in mossy fiber LTD.
2009-12-01 [Synapse 63(12) , 1060-8, (2009)] |
|
Behavioral evidence supporting a differential role for spina...
2002-09-01 [Neuropharmacology 43(3) , 319-26, (2002)] |
|
Enantioselective Synthesis of L-CCG-I.
2003-08-22 [J. Org. Chem. 68(17) , 6817-9, (2003)] |