Analyst (Cambridge UK)
2012-11-07
A colorimetric and fluorescent dual probe for specific detection of cysteine based on intramolecular nucleophilic aromatic substitution.
Limin Ma, Junhong Qian, Haiyu Tian, Minbo Lan, Weibing Zhang
Index: Analyst 137 , 5046-5050, (2012)
Full Text: HTML
Abstract
4-Nitro-1,8-naphthalic anhydride (NNA) was used to distinguish cysteine from homocysteine and other potentially interfering thiols through a novel sequential substitution mechanism. The discrimination involves a blue-fluorescent thioether formation via nucleophilic aromatic substitution of the nitro group by thiol, followed by a second intramolecular nucleophilic aromatic substitution of alkylthio with the amino group to give the green-fluorescent 4-amino derivative. NNA is highly selective towards Cys, and the detection limit of Cys by this method is 0.3 μM.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
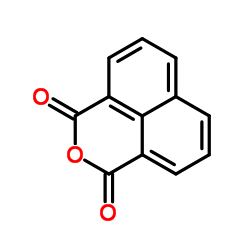 |
1,8-Naphthalic anhydride
CAS:81-84-5 |
C12H6O3 |
Related Articles:
More...
|
1,8-Naphthalic anhydride antidote enhances the toxic effects...
1991-01-01 [Res. Microbiol. 142(9) , 1005-12, (1991)] |
|
Sensitive enantiomeric separation of aliphatic and aromatic ...
1994-04-22 [J. Chromatogr. A. 666 , 485-491, (1994)] |
|
Differential induction of cytochrome P450-mediated triasulfu...
1995-12-01 [Plant Physiol. 109(4) , 1483-90, (1995)] |
|
A herbicide antidote (safener) induces the activity of both ...
1994-09-26 [FEBS Lett. 352(2) , 219-21, (1994)] |
|
Inosine protects against the development of diabetes in mult...
2003-01-01 [Mol. Med. 9(3-4) , 96-104, (2003)] |