| Structure | Name/CAS No. | Articles |
|---|---|---|
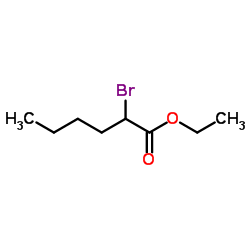 |
Ethyl 2-bromohexanoate
CAS:615-96-3 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethyl 2-bromohexanoate
CAS:615-96-3 |