Synthesis and dynamic NMR study of ketenimines derived from tert-butyl isocyanide, alkyl 2-arylamino-2-oxo-acetates, and dialkyl acetylenedicarboxylates.
Issa Yavari, Farough Nasiri, Horieh Djahaniani
Index: Mol. Divers. 8(4) , 431-5, (2004)
Full Text: HTML
Abstract
The adduct produced in the reaction between tert-butyl isocyanide and dialkyl acetylenedicarboxylates was trapped by alkyl 2-arylamino-2-oxo-acetates. When the aryl group is 2-methyl-6-nitrophenyl or 2,6-di-isopropylphenyl, the product exists as two stable rotamers at room temperature as a result of restricted rotation around the Ar-N single bond. When the aryl group is 1-naphthyl or 8-quinolinyl, dynamic NMR effects are observed in the 1H NMR spectra. The calculated free-energy of activation for interconversion of the rotational isomers in 1-naphthyl and 8-quinolinyl derivatives amounts to about 99+/-2 and 68.5+/-2 kJ mol(-1), respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
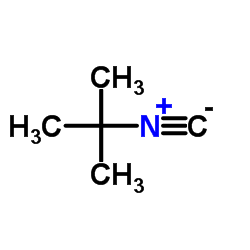 |
Tert-Butyl isocyanide
CAS:7188-38-7 |
C5H9N |
|
Optimization of the Ugi reaction using parallel synthesis an...
2008-01-01 [J. Vis. Exp. (21) , doi:10.3791/942, (2008)] |
|
Palladium-catalyzed synthesis of isocoumarins and phthalides...
2012-11-16 [J. Org. Chem. 77(22) , 10321-8, (2012)] |
|
Towards molecular diversity: dealkylation of tert-butyl amin...
2010-08-21 [Org. Biomol. Chem. 8(16) , 3631-4, (2010)] |
|
Strong carbon-surface dative bond formation by tert-butyl is...
2014-04-23 [J. Am. Chem. Soc. 136(16) , 5848-51, (2014)] |
|
Structural and spectroscopic studies of some copper(I) halid...
2008-04-07 [Dalton Trans. (13) , 1710-20, (2008)] |