Computational design of an enzyme catalyst for a stereoselective bimolecular Diels-Alder reaction.
Justin B Siegel, Alexandre Zanghellini, Helena M Lovick, Gert Kiss, Abigail R Lambert, Jennifer L St Clair, Jasmine L Gallaher, Donald Hilvert, Michael H Gelb, Barry L Stoddard, Kendall N Houk, Forrest E Michael, David Baker
Index: Science 329(5989) , 309-13, (2010)
Full Text: HTML
Abstract
The Diels-Alder reaction is a cornerstone in organic synthesis, forming two carbon-carbon bonds and up to four new stereogenic centers in one step. No naturally occurring enzymes have been shown to catalyze bimolecular Diels-Alder reactions. We describe the de novo computational design and experimental characterization of enzymes catalyzing a bimolecular Diels-Alder reaction with high stereoselectivity and substrate specificity. X-ray crystallography confirms that the structure matches the design for the most active of the enzymes, and binding site substitutions reprogram the substrate specificity. Designed stereoselective catalysts for carbon-carbon bond-forming reactions should be broadly useful in synthetic chemistry.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
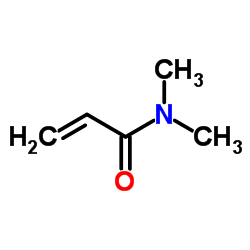 |
Acryloyldimethylamine
CAS:2680-03-7 |
C5H9NO |
|
Matrix-assisted refolding and purification of placenta-deriv...
2014-01-01 [Biotechnol. Appl. Biochem. 61(5) , 541-8, (2014)] |
|
Hydrogels as feeder-free scaffolds for long-term self-renewa...
2015-04-01 [J. Tissue Eng. Regen. Med. 9(4) , 375-88, (2015)] |
|
Detection of Peptide-based nanoparticles in blood plasma by ...
2015-01-01 [PLoS ONE 10 , e0126136, (2015)] |
|
Identification of multiple novel protein biomarkers shed by ...
2013-01-01 [PLoS ONE 8 , e60129, (2013)] |
|
Exploring Carbon Nanomaterial Diversity for Nucleation of Pr...
2009-07-01 [Sci. Rep. 6 , 20053, (2016)] |