Developmentally regulated glycosylation of dopamine transporter.
A P Patel, C Cerruti, R A Vaughan, M J Kuhar
Index: Brain Res. Dev. Brain Res. 83(1) , 53-8, (1994)
Full Text: HTML
Abstract
The dopamine transporter (DAT) in rat striatum was examined during postnatal development and aging after photolabeling with [125I]DEEP. The DAT-[125I]DEEP protein complex from adult rats (2 months) appeared as a broad diffuse band in SDS-PAGE gels with average apparent molecular mass of about 80,000 Da as previously found. However, the molecular mass was lower at birth (day 0) and at postnatal ages 4 and 14 days. In aged rats (104 weeks), the molecular mass was slightly higher than that found in young adults (60 days). In binding experiments with [3H]BTCP, there were age-related differences in Kd and Bmax with decreases in both Kd and Bmax found in aged rats. Treatment of photolabeled membranes with neuraminidase caused a reduction in DAT molecular mass, but age-related differences were maintained. Treatment with N-glycanase greatly reduced or eliminated the age-related differences. Several DAT peptide-specific polyclonal antibodies immunoprecipitated DAT-[125I]DEEP protein complex at different developmental ages. Taken together, these results suggest differential glycosylation of rat DAT occurs during postnatal development and aging; the increase is due to increases in the N-linked sugars rather than changes in either sialic acid content or the polypeptide.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
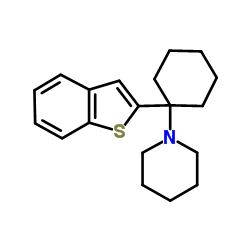 |
BTCP HCl
CAS:112726-66-6 |
C19H25NS |
|
Studies of the biogenic amine transporters. III. Demonstrati...
1994-03-01 [J. Pharmacol. Exp. Ther. 268 , 1462-1475, (1994)] |
|
MK-801, phencyclidine (PCP), and PCP-like drugs increase bur...
1993-02-01 [Synapse 13(2) , 108-16, (1993)] |
|
N-[1-(2-benzo[b]thiophenyl)Cyclohexyl]- piperidine (BTCP) ex...
2000-09-01 [Biol. Psychiatry 23(3) , 316-25, (2000)] |
|
Thermodynamics of the binding of BTCP (GK 13) and related de...
1994-08-16 [Eur. J. Pharmacol. 268(3) , 357-63, (1994)] |
|
Alterations in the dopaminergic receptor system after chroni...
1993-08-01 [Synapse 14(4) , 314-23, (1993)] |