Recent advances in enzymatic and chemical deracemisation of racemic compounds.
Michał Rachwalski, Niek Vermue, Floris P J T Rutjes
Index: Chem. Soc. Rev. 42(24) , 9268-9282, (2013)
Full Text: HTML
Abstract
Deracemisation of racemic compounds is still the most important strategy to produce optically pure compounds despite many recent advances in asymmetric synthesis. Especially deracemisation approaches that give rise to single enantiomers are preferred, which can be achieved either by invoking organocatalysts, metal complexes or enzymes - leading to dynamic kinetic resolution - or by applying other deracemisation techniques based on stereoinversion or enantioconvergence. In this tutorial review, we will provide an overview of new, recently developed approaches that lead to the important goal of creating organic compounds of single chirality.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
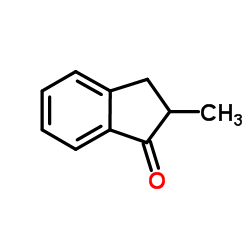 |
2-Methyl-indanone
CAS:17496-14-9 |
C10H10O |
|
The O-acylation of ketone enolates by allyl 1H-imidazole-1-c...
2007-11-23 [J. Org. Chem. 72(24) , 9372-5, (2007)] |
|
Amino alcohol-mediated enantioselective syntheses of α-...
[Tetrahedron Asymmetry 25(9) , 697-704, (2014)] |
|
Structural effects in the Pd-induced enantioselective deprot...
[Tetrahedron Asymmetry 18(24) , 2859-2868, (2007)] |
|
Heck-type reactions of allylic alcohols: Part IV:(2-Substitu...
[J. Mol. Catal. A: Chem. 283(1) , 140-45, (2008)] |
|
Cyclophanes. 9. anti-[2.2](2, 6) Azulenophane. Synthesis and...
[J. Am. Chem. Soc. 99(11) , 3797-3805, (1977)] |