Unprecedented head-to-head conformers of d(GpG) bound to the antitumor active compound tetrakis (mu-carboxylato)dirhodium(II,II).
Helen T Chifotides, Karl M Koshlap, Lisa M Pérez, Kim R Dunbar
Index: J. Am. Chem. Soc. 125(35) , 10703-13, (2003)
Full Text: HTML
Abstract
The N7/O6 equatorial binding interactions of the antitumor active complex Rh(2)(OAc)(4)(H(2)O)(2) (OAc(-) = CH(3)CO(2)(-)) with the DNA fragment d(GpG) have been unambiguously determined by NMR spectroscopy. Previous X-ray crystallographic determinations of the head-to-head (HH) and head-to-tail (HT) adducts of dirhodium tetraacetate with 9-ethylguanine (9-EtGH) revealed unprecedented bridging N7/O6 guanine nucleobases that span the Rh-Rh bond. The absence of N7 protonation at low pH and the notable increase in the acidity of N1-H (pK(a) approximately 5.7 as compared to 8.5 for N7 only bound platinum adducts), suggested by the pH dependence titrations of the purine H8 (1)H NMR resonances for Rh(2)(OAc)(2)(9-EtG)(2) and Rh(2)(OAc)(2-)[d(GpG)],are consistent with bidentate N7/O6 binding of the guanine nucleobases. The pK(a) values estimated for N1-H (de)protonation, from the pH dependence studies of the C6 and C2 (13)C NMR resonances for the Rh(2)(OAc)(2)(9-EtG)(2) isomers, concur with those derived from the H8 (1)H NMR resonance titrations. Comparison of the (13)C NMR resonances of C6 and C2 for the dirhodium adducts Rh(2)(OAc)(2)(9-EtG)(2) and Rh(2)(OAc)(2)[d(GpG)] with the corresponding resonances of the unbound ligands [at pH 7.0 for 9-EtGH and pH 8.0 for d(GpG)], shows substantial downfield shifts of Deltadelta approximately 11.0 and 6.0 ppm for C6 and C2, respectively; the latter shifts reflect the effect of O6 binding to the dirhodium centers and the ensuing enhancement in the acidity of N1-H. Intense H8/H8 ROE cross-peaks in the 2D ROESY NMR spectrum of Rh(2)(OAc)(2)[d(GpG)] indicate head-to-head arrangement of the guanine bases. The Rh(2)(OAc)(2)[d(GpG)] adduct exhibits two major right-handed conformers, HH1 R and HH2 R, with HH1 R being three times more abundant than the unusual HH2 R. Complete characterization of both adducts revealed repuckering of the 5'-G sugar rings to C3'-endo (N-type), retention of C2'-endo (S-type) conformation for the 3'-G sugar rings, and anti orientation with respect to the glycosyl bonds. The structural features obtained for Rh(2)(OAc)(2))[d(GpG)] by means of NMR spectroscopy are very similar to those for cis-[Pt(NH(3))(2))[d(GpG)]] and corroborate molecular modeling studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
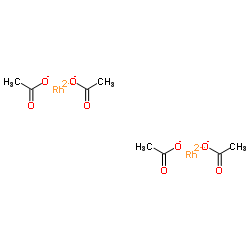 |
Dirhodium tetraacetate
CAS:15956-28-2 |
C8H12O8Rh2 | |
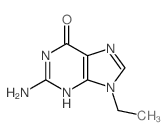 |
9-ethylguanine
CAS:879-08-3 |
C7H9N5O |
|
Rhodium acetate/base-catalyzed N-silylation of indole deriva...
2012-09-25 [Chem. Commun. (Camb.) 48(74) , 9269-71, (2012)] |
|
One-pot protocol to functionalized benzopyrrolizidine cataly...
2015-01-02 [Org. Lett. 17(1) , 66-9, (2015)] |
|
Distal renal tubular acidosis associated with anion exchange...
2007-06-01 [Am. J. Kidney Dis. 49(6) , 841-850.e1, (2007)] |
|
Interaction of tetra-mu-acetatodirhodium(II) with human seru...
1995-04-01 [J. Inorg. Biochem. 58(1) , 69-77, (1995)] |
|
Vinyl diazophosphonates as precursors to quaternary substitu...
2011-02-18 [Org. Lett. 13(4) , 700-2, (2011)] |