| Structure | Name/CAS No. | Articles |
|---|---|---|
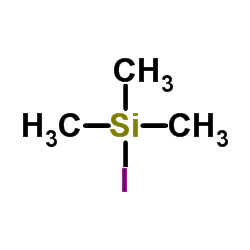 |
Trimethylsilyl iodide
CAS:16029-98-4 |
Z Tocik, R A Earl, J Beránek
Index: Nucleic Acids Res. 8 , 4755, (1980)
Full Text: HTML
1-O-Acetyl-2,3,5-tri-O-benzoyl-beta-D-ribofuranose (I) was reacted with iodotrimethylsilane (II) and the product, the glycosyl iodide, was coupled with silylated uracil to afford 1-(2,3,5-tri-O-benzoyl-beta-D-ribofuranosyl)uracil (III; 89%), with silyated cytosine to afford, on subsequent acetylation, 1-(2,3,5-tri-O-benzoyl-beta-0D-ribofuranosyl)-4-acetamido-2-(1H)-pyrimidinone (IVb; 81%), and with chloromercuri-N-benzoyl-adenine to afford Va and on subsequent debenzoylation, adenosine (Vb; 49%).
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Trimethylsilyl iodide
CAS:16029-98-4 |
C3H9ISi |
|
Occurrence, removal, and fate of progestogens, androgens, es...
2014-11-01 [Environ. Sci. Pollut. Res. Int. 21(22) , 12898-908, (2014)] |
|
Validation of an enzyme immunoassay for the measurement of f...
2014-09-15 [Gen. Comp. Endocrinol. 206 , 166-77, (2014)] |
|
[Determination of anabolic steroid hormones in animal muscle...
2006-01-01 [Se Pu 24(1) , 19-22, (2006)] |
|
[Tetrahedron Lett. 35 , 5445, (1994)] |
|
[Tetrahedron Lett. 45 , 3607, (2004)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved