Nickel-catalyzed Suzuki-Miyaura reaction of aryl fluorides.
Mamoru Tobisu, Tian Xu, Toshiaki Shimasaki, Naoto Chatani
Index: J. Am. Chem. Soc. 48th ed., 133 , 19505-19511, (2011)
Full Text: HTML
Abstract
Two protocols for the nickel-catalyzed cross-coupling of aryl fluorides with aryl boronic esters have been developed. The first employs metal fluoride cocatalysts, such as ZrF(4) and TiF(4), which enable Suzuki-Miyaura reactions of aryl fluorides bearing electron-withdrawing (ketones, esters, and CF(3)), aryl and alkenyl groups as well as those comprising fused aromatic rings, such as fluoronaphthalenes and fluoroquinolines. The second protocol employs aryl fluorides bearing ortho-directing groups, which facilitate the difficult C-F bond activation process via cyclometalation. N-heterocycles, such as pyridines, quinolines, pyrazoles, and oxazolines, can successfully promote cross-coupling with an array of organoboronic esters. A study into the substituent effects with respect to both coupling components has provided fundamental insights into the mechanism of the nickel-catalyzed cross-coupling of aryl fluorides.© 2011 American Chemical Society
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
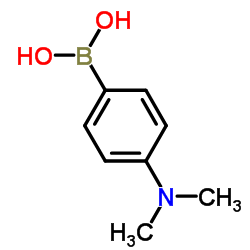 |
4-(Dimethylamino)phenylboronic acid
CAS:28611-39-4 |
C8H12BNO2 |
|
Synthesis and photophysical investigation of a series of pus...
2012-04-20 [J. Org. Chem. 8th ed., 77 , 4087-4096, (2012)] |
|
Dramatic Effect of the Gelling Cation on the Catalytic Perfo...
[Chem. Mater. 8th ed., 24 , 1505-1510, (2012)] |
|
Organic dyes incorporating the cyclopentadithiophene moiety ...
[Dyes and Pigments 3rd ed., 92 , 1292-1299, (2012)] |
|
Rapid access to 4-substituted-pyrones and 2(5H)-furanones vi...
[Tetrahedron 38th ed., 67 , 7258-7262, (2011)] |
|
Chiral allene-containing phosphines in asymmetric catalysis.
2011-11-16 [J. Am. Chem. Soc. 45th ed., 133 , 18066-18069, (2011)] |