Identification of the substrate interaction site in the N-terminal membrane anchor segment of thromboxane A2 synthase by determination of its substrate analog conformational changes using high resolution NMR technique.
S P So, D Li, K H Ruan
Index: J. Biol. Chem. 275(52) , 40679-85, (2000)
Full Text: HTML
Abstract
The present studies describe an investigation for the interaction of N-terminal membrane anchor domain of thromboxane A(2) synthase (TXAS) with its substrate analog in a membrane-bound environment using the two-dimensional NMR technique. TXAS and prostaglandin I(2) synthase (PGIS), respectively, convert the same substrate, prostaglandin H(2) (PGH(2)), to thromboxane A(2) and prostaglandin I(2), which have opposite biological functions. Our topology studies have indicated that the N-terminal region of TXAS has a longer N-terminal endoplasmic reticulum (ER) membrane anchor region compared with the same segment proposed for PGIS. The differences in their interaction with the ER membrane may have an important impact to facilitate their common substrate, PGH(2), across the membrane into their active sites from the luminal to the cytoplasmic side of the ER. To test this hypothesis, we first investigated the interaction of the TXAS N-terminal membrane anchor domain with its substrate analog. A synthetic peptide corresponding to the N-terminal membrane anchor domain (residues 1-35) of TXAS, which adopted a stable helical structure and exhibited a membrane anchor function in the membrane-bound environment, was used to interact with a stable PGH(2) analog,. High resolution two-dimensional NMR experiments, NOESY and TOCSY, were performed to solve the solution structures of in a membrane-mimicking environment using dodecylphosphocholine micelles. Different conformations were clearly observed in the presence and absence of the TXAS N-terminal membrane anchor domain. Through combination of the two-dimensional NMR experiments, completed (1)H NMR assignments of were obtained, and the data were used to construct three-dimensional structures of in H(2)O and dodecylphosphocholine micelles, showing the detailed conformation change upon the interaction with the membrane anchor domain. The observation supported the presence of a substrate interaction site in the N-terminal region. The combination of the structural information of and was able to simulate a solution structure of the unstable TXAS and PGIS substrate, PGH(2).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
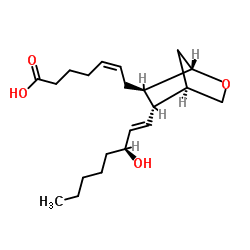 |
U 44069
CAS:56985-32-1 |
C21H34O4 |
|
Endothelin, PAF and thromboxane A2 in allergic pulmonary hyp...
2007-05-01 [Prostaglandins Leukot. Essent. Fatty Acids 76(5) , 299-308, (2007)] |
|
Glibenclamide inhibits agonist-induced vasoconstriction of p...
2006-01-01 [Placenta 27(6-7) , 660-8, (2006)] |
|
Substrate binding is the rate-limiting step in thromboxane s...
2001-05-04 [J. Biol. Chem. 276(18) , 14737-43, (2001)] |
|
On the regulation of tone in vasa vasorum.
1999-01-01 [Cardiovasc. Res. 41(1) , 237-45, (1999)] |
|
In vivo effects of a novel thromboxane A2/prostaglandin H2 (...
1995-02-01 [J. Pharmacol. Exp. Ther. 272(2) , 799-807, (1995)] |