Synergistic induction of apoptosis in human leukemia cells (U937) exposed to bryostatin 1 and the proteasome inhibitor lactacystin involves dysregulation of the PKC/MAPK cascade.
J A Vrana, S Grant
Index: Blood 97(7) , 2105-14, (2001)
Full Text: HTML
Abstract
Cotreatment with a minimally toxic concentration of the protein kinase C (PKC) activator (and down-regulator) bryostatin 1 (BRY) induced a marked increase in mitochondrial dysfunction and apoptosis in U937 monocytic leukemia cells exposed to the proteasome inhibitor lactacystin (LC). This effect was blocked by cycloheximide, but not by alpha-amanitin or actinomycin D. Qualitatively similar interactions were observed with other PKC activators (eg, phorbol 12-myristate 13-acetate and mezerein), but not phospholipase C, which does not down-regulate the enzyme. These events were examined in relationship to functional alterations in stress (eg, SAPK, JNK) and survival (eg, MAPK, ERK) signaling pathways. The observations that LC/BRY treatment failed to trigger JNK activation and that cell death was unaffected by a dominant-interfering form of c-JUN (TAM67) or by pretreatment with either curcumin or the p38/RK inhibitor, SB203580, suggested that the SAPK pathway was not involved in potentiation of apoptosis. In marked contrast, perturbations in the PKC/Raf/MAPK pathway played an integral role in LC/BRY-mediated cell death based on evidence that pretreatment of cells with bisindolylmaleimide I, a selective PKC inhibitor, or geldanamycin, a benzoquinone ansamycin, which destabilizes and depletes Raf-1, markedly suppressed apoptosis. Furthermore, ERK phosphorylation was substantially prolonged in LC/BRY-treated cells compared to those exposed to BRY alone, and pretreatment with the highly specific MEK inhibitors, PD98059, U0126, and SL327, opposed ERK activation while protecting cells from LC/BRY-induced lethality. Together, these findings suggest a role for activation and/or dysregulation of the PKC/MAPK cascade in modulation of leukemic cell apoptosis following exposure to the proteasome inhibitor LC. (Blood. 2001;97:2105-2114)
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
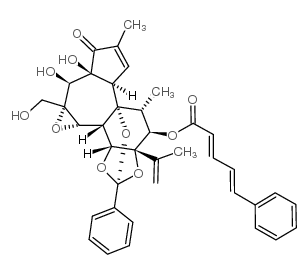 |
2,4-Pentadienoic acid,5-phenyl-,(2S,3aR,3bS,3cS,4aR,5S,5aS,8aR,8bR,9R,10R,10aS)-3a,3b,3c,4a,5,5a,8a,9,10,10a-decahydro-5,5a-dihydroxy-4a-(hydroxymethyl)-7,9-dimethyl-10a-(1-methylethenyl)-6-oxo-2-phen
CAS:34807-41-5 |
C38H38O10 |
|
Models of acute inflammation in the ear.
2003-01-01 [Methods Mol. Biol. 225 , 129-37, (2003)] |
|
Gateway synthesis of daphnane congeners and their protein ki...
2011-08-01 [Nature Chemistry 3(8) , 615-9, (2011)] |
|
Analysis of daphnane orthoesters in poisonous Australian pim...
2010-06-23 [J. Agric. Food Chem. 58(12) , 7482-7, (2010)] |
|
E6201, a novel kinase inhibitor of mitogen-activated protein...
2010-10-01 [J. Pharmacol. Exp. Ther. 335(1) , 23-31, (2010)] |
|
An asymmetric synthesis of aza analogues of the tricyclic sk...
2003-02-07 [J. Org. Chem. 68(3) , 792-8, (2003)] |