Characterization of 2'-deoxycytidine and 2'-deoxyuridine adducts formed in reactions with acrolein and 2-bromoacrolein.
A Chenna, C R Iden
Index: Chem. Res. Toxicol. 6(3) , 261-8, (1993)
Full Text: HTML
Abstract
The products of the reaction of the mutagenic aldehydes, acrolein and 2-bromoacrolein, with 2'-deoxycytidine and 2'-deoxyuridine have been determined. These products, formed at physiological conditions, were isolated by reverse-phase HPLC and characterized by UV, 1H NMR, fast atom bombardment MS, electrospray MS, and chemical transformation. The reaction of 2'-deoxycytidine with acrolein and 2-bromoacrolein produced the exocyclic compounds 3-(2'-deoxyribosyl)-7,8,9-trihydro-7-hydroxypyrimido[3,4- c]pyrimidin-2-one and 3-(2'-deoxyribosyl)-7,8,9-trihydro-7-hydroxy-8-bromopyrimido [3,4-c] pyrimidin-2-one, respectively. In addition to the chiral centers of deoxyribose, one new chiral center was formed from C-1 of acrolein and two new chiral centers were formed from C-1 and C-2 of 2-bromoacrolein, creating a mixture of diastereomers for each product. These compounds are not stable in basic solution and undergo ring opening and hydrolytic deamination, resulting in 2'-deoxyuridine adducts. The N3-alkylated 2'-deoxyuridines were also synthesized by permitting 2'-deoxyuridine to react with 2-bromoacrolein and acrolein. An unstable intermediate, N3-(2"-bromo-3"-oxopropyl)-2'-deoxyuridine, was also isolated and characterized from the reaction with 2-bromoacrolein. The reaction of 2'-deoxyuridine with acrolein gave N3-(3"-oxopropyl)-2'-deoxyuridine as the major product, which was reduced to its corresponding alcohol with NaBH4. Reactions of 2'-deoxycytidine with 2-bromoacrolein and acrolein proceed most rapidly at acidic or neutral pH; however, 2'-deoxyuridine reacts most rapidly at neutral or basic pH.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
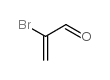 |
2-BROMOACRYLALDEHYDE
CAS:14925-39-4 |
C3H3BrO |
|
Metabolism and genotoxicity of the halogenated alkyl compoun...
1994-12-01 [Hum. Exp. Toxicol. 13(12) , 861-5, (1994)] |
|
Genotoxicity of 2-halosubstituted enals and 2-chloroacryloni...
1994-10-01 [Mutat. Res. 322(4) , 321-8, (1994)] |
|
Formation of thymidine, cyclic deoxyguanosine and cyclic deo...
1994-01-01 [IARC Sci. Publ. (125) , 449-52, (1994)] |
|
Formation of cyclic 1,N2-propanodeoxyguanosine and thymidine...
1989-11-15 [Cancer Res. 49(22) , 6174-9, (1989)] |
|
Formation of thymidine adducts and cross-linking potential o...
1992-01-01 [Mutagenesis 7(1) , 19-24, (1992)] |