[Degradation of diuron by persulfate oxidation activated by EDTA-ferrous ion in aqueous system].
Jin-Feng Zhang, Xi Yang, Wei Zheng, Ling-Ren Kong, Lian-Hong Wang
Index: Huan Jing Ke Xue 29(5) , 1239-43, (2008)
Full Text: HTML
Abstract
The method of diuron [3-(3,4-dichlorophenyl)-1, 1-dimethylurea] degradation by persulfate oxidation activated by EDTA-ferrous ion in aqueous system was conducted. Based on both of the degradation performance and the operating costs, optimal reaction condition was proposed. Operating at K2S2O8 initial concentration 2.0 mmol x L(-1), Fe(II) initial concentration 1.0 mmol x L(-1), EDTA initial concentration 0.5 mmol x L(-1), reaction time 300 min and pH = 7.0, about 67.6% of 0.1 mmol x L(1) diuron was degradation. Hydroxyl radicals and sulfate radicals produced in the system were determined by molecular probes (ethanol and tert-butanol) methods. The degradation products of diuron were identified with LC/MS methods and the degradation pathways of diuron were discussed.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
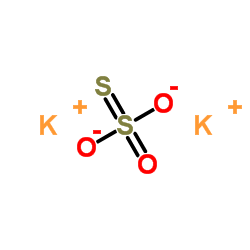 |
Potassium thiosulfate
CAS:10294-66-3 |
K2O3S2 |
|
Compounds of glycine with metal sulfates and thiosulfates: g...
2006-01-01 [Acta Crystallogr. C 62(Pt 1) , m22-6, (2006)] |
|
Separation, preconcentration and determination of silver ion...
2006-01-16 [J. Hazard. Mater. 128(1) , 67-72, (2006)] |
|
Effect of plastic tarps over raised-beds and potassium thios...
2008-06-01 [Chemosphere 72(4) , 558-63, (2008)] |
|
Improved method to calculate the antibody avidity index.
2007-01-01 [J. Clin. Lab Anal. 21(3) , 201-6, (2007)] |
|
[Resistance of Aujeszky disease viruses, present in the bloo...
1984-05-01 [Vet. Med. (Praha.) 29(5) , 279-85, (1984)] |