Class II α-mannosidase fromAspergillus fischeri: Energetics of catalysis and inhibition
K.S. Shashidhara, Sushama M. Gaikwad, K.S. Shashidhara, Sushama M. Gaikwad
Index: Int. J. Biol. Macromol. 44(1) , 112-5, (2009)
Full Text: HTML
Abstract
Energetics of the catalysis of Class II α-mannosidase (E.C.3.2.1.24) from Aspergillus fischeri was studied. The enzyme showed K cat/ K m for Man (α1-3) Man, Man (α1-2) Man and Man (α1-6) Man as 7488, 5376 and 3690 M −1 min −1, respectively. The activation energy, E a was 15.14, 47.43 and 71.21 kJ/mol for α1-3, α1-2 and α1-6 linked mannobioses, respectively, reflecting the energy barrier in the hydrolysis of latter two substrates. The enzyme showed K cat/ K m as 3.56 × 10 5 and 4.61 × 10 5 M −1 min −1 and E a as 38.7 and 8.92 kJ/mol, towards pNPαMan and 4-MeUmbαMan, respectively. Binding of Swainsonine to the enzyme is stronger than that of 1-deoxymannojirimycin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
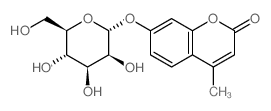 |
4-methylumbelliferyl beta-D-mannopyranoside
CAS:28541-83-5 |
C16H18O8 |
|
Binding of manno-oligosaccharides to concanavalin A. Substit...
1980-01-01 [Eur. J. Biochem. 103(2) , 307-12, (1980)] |
|
Enzyme studies on human and snail beta-mannosidase using a f...
1992-04-01 [Int. J. Biochem. 24(4) , 669-73, (1992)] |
|
Kinetics of interaction of some alpha- and beta-D-monosaccha...
1980-09-01 [Biochim. Biophys. Acta 631(3) , 428-38, (1980)] |
|
The putative role of members of the CEA-gene family (CEA, NC...
1991-04-01 [Zentralbl. Bakteriol. 275(1) , 118-22, (1991)] |
|
Binding of methylumbelliferyl mannoside to concanavalin A un...
1984-10-09 [Biochim. Biophys. Acta 790(1) , 87-90, (1984)] |