| Structure | Name/CAS No. | Articles |
|---|---|---|
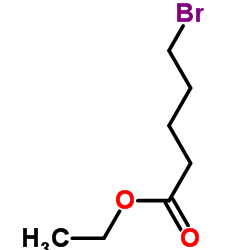 |
Ethyl 5-Bromovalerate
CAS:14660-52-7 |
| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethyl 5-Bromovalerate
CAS:14660-52-7 |