Organic & Biomolecular Chemistry
2006-05-21
Reactivity of cyclic sulfamidates towards sulfur-stabilised enolates. Stereocontrolled synthesis of functionalised lactams.
John F Bower, Suda Chakthong, Jakub Svenda, Andrew J Williams, Ron M Lawrence, Peter Szeto, Timothy Gallagher
Index: Org. Biomol. Chem. 4(10) , 1868-77, (2006)
Full Text: HTML
Abstract
A structurally representative series of 1,2- and 1,3-cyclic sulfamidates react with enolates derived from methyl alpha-phenylthioacetate 9b to give 5- and 6-substituted alpha-phenylthio lactams 20-24. These products provide, via the corresponding sulfoxides, an entry to alpha,beta-unsaturated lactams e.g. 12, 27, 29 and their alpha-phenylthio analogues e.g. 26 and 30. With the enantiomerically pure 1,2-cyclic sulfamidates 10, 15 and 17, these reactions all proceed with no detectable loss of stereochemical integrity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
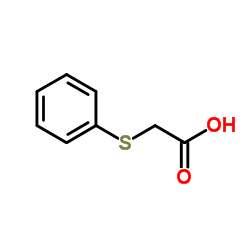 |
Phenyl thioacetic acid
CAS:103-04-8 |
C8H8O2S |
Related Articles:
More...
|
Density functional theory calculations of structure, FT-IR a...
2011-10-15 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 81(1) , 236-41, (2011)] |
|
Evaluation of a rapid differentiation test for the Mycobacte...
2005-02-01 [Int. J. Tuberc. Lung Dis. 9(2) , 206-9, (2005)] |
|
Identification of two rat liver proteins with paraoxonase ac...
1999-05-14 [Chem. Biol. Interact. 119-120 , 263-75, (1999)] |
|
Hydrolysis of oxo- and thio-esters by human butyrylcholinest...
2007-01-01 [Biochim. Biophys. Acta 1774(1) , 16-34, (2007)] |
|
Ascorbic acid-dependent turnover and reactivation of 2,4-dic...
1998-03-03 [Biochemistry 37(9) , 3035-42, (1998)] |