Short-term toxicity study of ST-20 (NSC-741804) by oral gavage in Sprague-Dawley rats.
Pramod S Terse, Jerry D Johnson, Michael A Hawk, Glenn D Ritchie, Michael J Ryan, Daphne Y Vasconcelos, Denise A Contos, Susan P Perrine, James O Peggins, Joseph E Tomaszewski
Index: Toxicol. Pathol. 39(4) , 614-22, (2011)
Full Text: HTML
Abstract
ST-20 (sodium 2,2-dimethylbutyrate) is a potential therapeutic agent for treatment of β-thalassemia and sickle cell disease. A subchronic oral toxicity study was conducted in Sprague-Dawley rats (10/sex/dose) at gavage dosages of 0 (vehicle control), 200, 600, or 1,000 mg/kg, once daily for up to 15 days followed by a 14-day recovery. Ataxia (females), rough coat/thin appearance (males), and decreased body weights were observed at 1,000 mg/kg. Functional observational battery (FOB) deficits were observed more frequently in females and included decreased body tone, rectal temperature, emotional reactivity, neuromotor-neuromuscular activity (as exhibited by a deficit in visual/tactile placing accuracy, ataxia, hind limb dragging, and decreased grip strength), and rearing. ST-20 caused a decrease in WBC/RBC counts and RBC parameters; increase in reticulocytes and red cell inclusion bodies; decrease in total protein, globulin, and glucose; and increase in AG ratio. Micronucleated polychromatic erythrocytes of the bone marrow increased significantly in males at 1,000 mg/kg. Mean liver and kidney weights increased, and hepatocellular hypertrophy was observed in males at 1,000 mg/kg. Toxicologic findings were fully recovered during the 14-day recovery period. In conclusion, the no-observed adverse effect level for FOB and general toxicity was 200 mg/kg following gavage administration of ST-20 for up to 15 consecutive days.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
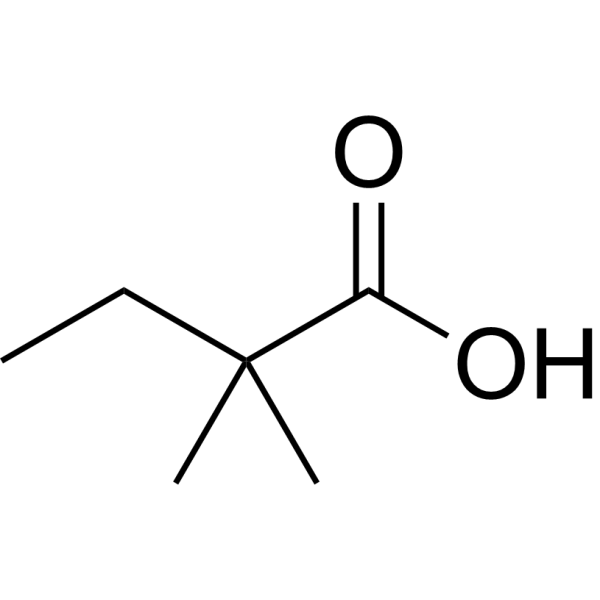 |
2,2-Dimethylbutanoic acid
CAS:595-37-9 |
C6H12O2 |
|
Liquid chromatography-mass spectrometric assay for quantitat...
2008-02-01 [J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 862(1-2) , 168-74, (2008)] |
|
[Chemical components of essential oils from the herb of Ligu...
2003-07-01 [Zhongguo Zhong Yao Za Zhi 28(7) , 627-9, (2003)] |
|
A randomized phase I/II trial of HQK-1001, an oral fetal glo...
2013-05-01 [Br. J. Haematol. 161(4) , 587-93, (2013)] |
|
A phase 1/2 trial of HQK-1001, an oral fetal globin inducer,...
2012-11-01 [Am. J. Hematol. 87(11) , 1017-21, (2012)] |
|
Evaluation of safety and pharmacokinetics of sodium 2,2 dime...
2011-08-01 [J. Clin. Pharmacol. 51(8) , 1186-94, (2011)] |