Synthesis and oligomerization of Fmoc/Boc-protected PNA monomers of 2,6-diaminopurine, 2-aminopurine and thymine.
André H St Amant, Robert H E Hudson
Index: Org. Biomol. Chem. 10(4) , 876-81, (2012)
Full Text: HTML
Abstract
A Boc-protecting group strategy for Fmoc-based PNA (peptide nucleic acid) oligomerization has been developed for thymine, 2,6-diaminopurine (DAP) and 2-aminopurine (2AP). The monomers may be used interchangeably with standard Fmoc PNA monomers. The DAP monomer was incorporated into a PNA and was found to selectively bind to T (ΔT(m)≥ +6 °C) in a complementary DNA strand. The 2AP monomer showed excellent discrimination of T (ΔT(m)≥ +12 °C) over the other nucleobases. 2AP also acted as a fluorescent probe of the PNA:DNA duplexes and displayed fluorescence quenching dependent on the opposite base.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
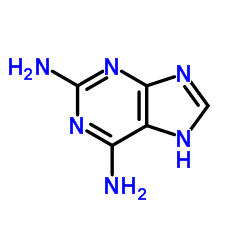 |
2,6-Diaminopurine
CAS:1904-98-9 |
C5H6N6 |
|
Effect of substituents on the excited-state dynamics of the ...
2010-01-01 [Phys. Chem. Chem. Phys. 12(20) , 5375-88, (2010)] |
|
Formation and helicity control of ssDNA templated porphyrin ...
2013-02-01 [Chem. Commun. (Camb.) 49(10) , 1020-2, (2013)] |
|
Simulations of A-RNA duplexes. The effect of sequence, solut...
2012-08-23 [J. Phys. Chem. B 116(33) , 9899-916, (2012)] |
|
Biotransformation of 2,6-diaminopurine nucleosides by immobi...
2012-01-01 [Biotechnol. Prog. 28(5) , 1251-6, (2012)] |
|
Recombination R-triplex: H-bonds contribution to stability a...
2006-01-01 [Nucleic Acids Res. 34(11) , 3239-45, (2006)] |