Inorganic Chemistry
2008-03-03
Kinetic evidence for high reactivity of 3-nitrophenylboronic acid compared to its conjugate boronate ion in reactions with ethylene and propylene glycols.
Chiaki Miyamoto, Kazunori Suzuki, Satoshi Iwatsuki, Masahiko Inamo, Hideo D Takagi, Koji Ishihara
Index: Inorg. Chem. 47(5) , 1417-9, (2008)
Full Text: HTML
Abstract
The rate constants for a boronate ion were determined for the first time using the reaction systems of 3-nitrophenylboronic acid (3-NO2PhB(OH)2) with ethylene glycol (EG) and propylene glycol (PG) in an alkaline solution: the rate constants (25 degrees C, I = 0.10 M) for the reactions of 3-NO2PhB(OH)3- are 1.2 M(-1) s(-1) (EG) and 1.5 M(-1) s(-1) (PG), which are at least 10(3) times smaller than those for the reactions of 3-NO 2PhB(OH)2 [1.0 x 10(4) M(-1) s(-1) (EG) and 5.8 x 10(3) M(-1) s(-1) (PG)].
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
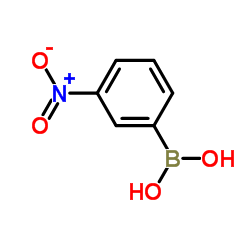 |
3-Nitrophenylboronic acid
CAS:13331-27-6 |
C6H6BNO4 |
Related Articles:
More...
|
Boronate affinity-assisted MEKC separation of highly hydroph...
2015-03-01 [Electrophoresis 36(5) , 784-95, (2015)] |
|
A pharmacological MRI assessment of dizocilpine (MK-801) in ...
2008-10-17 [Neurosci. Lett. 444(1) , 42-7, (2008)] |
|
Boronic acid catalyzed ene carbocyclization of acetylenic di...
2010-04-07 [Chem. Commun. (Camb.) 46 , 2191, (2010)] |
|
Nitrophenylboronic acids as highly chemoselective probes to ...
2011-11-09 [J. Agric. Food Chem. 59(21) , 11403-6, (2011)] |
|
Direct analysis of polyols using 3-nitrophenylboronic acid i...
2010-10-01 [Anal. Bioanal. Chem 398(3) , 1349-56, (2010)] |