Michael additions versus cycloaddition condensations with ethyl nitroacetate and electron-deficient olefins.
Elena Trogu, Francesco De Sarlo, Fabrizio Machetti
Index: Chemistry 15(32) , 7940-8, (2009)
Full Text: HTML
Abstract
Ethyl nitroacetate (1) reacts with electron-poor olefins in the presence of a base to give either the Michael adducts 3 or the isoxazoline cycloadducts 4, resulting from water elimination. The proportions of the two products depend on the reaction conditions and change in the course of the process. Kinetic profiles for the two reactions show that the cycloaddition-condensations require long induction times that dramatically decrease upon addition of a copper salt to the catalytic system: the drops in the induction time cause increases in the proportion of cycloadducts 4, which are often the sole reaction products. This is the first report on the selective formation of products 3 and 4 from primary nitro compounds through modulation of the catalytic system.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
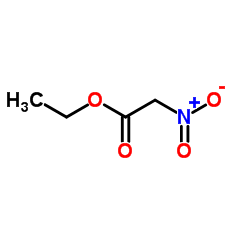 |
ETHYL NITROACETATE
CAS:626-35-7 |
C4H7NO4 |
|
[Experimental establishment of the maximum permissible conce...
1980-07-01 [Gig. Tr. Prof. Zabol. (7) , 49-51, (1980)] |
|
Synthesis and biological evaluation of DL-4,4-difluoroglutam...
1996-01-05 [J. Med. Chem. 39(1) , 66-72, (1996)] |
|
Formation of γ-oxoacids and 1H-pyrrol-2(5H)-ones from α,β-un...
2010-11-05 [J. Org. Chem. 75(21) , 7435-8, (2010)] |
|
Facile synthesis of alpha,alpha-diisobutylglycine and anchor...
2003-12-12 [J. Org. Chem. 68(25) , 9854-7, (2003)] |
|
Functionalization of pyrimidine and purine nucleosides at C4...
2005-01-01 [Nucleosides Nucleotides Nucleic Acids 24(5-7) , 1043-6, (2005)] |