A rapid route to aminocyclopropanes via carbamatoorganozinc carbenoids.
Shingo Ishikawa, Tom D Sheppard, Jarryl M D'Oyley, Akio Kamimura, William B Motherwell
Index: Angew. Chem. Int. Ed. Engl. 52(38) , 10060-3, (2013)
Full Text: HTML
Abstract
Easy as 1,2,3: Reaction of methyl carbamate, triethyl orthoformate, and readily available alkenes provides a highly practical preparation of protected aminocyclopropanes. The reaction proceeds with preferential cis addition to alkenes, and cleavage of the methyl carbamate gives the free aminocyclopropanes as their HI salts.© 2013 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
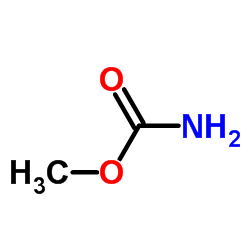 |
Methyl carbamate
CAS:598-55-0 |
C2H5NO2 |
|
The dissociation chemistry of ionized methyl carbamate and i...
2012-01-01 [Eur. J. Mass Spectrom. (Chichester, Eng.) 18(2) , 149-59, (2012)] |
|
Formaldehyde, glyoxal, urethane, methyl carbamate, 2,3-butan...
1992-11-16 [Mutat. Res. 270(2) , 151-66, (1992)] |
|
Thiadiazole carbamates: potent inhibitors of lysosomal acid ...
2010-07-22 [J. Med. Chem. 53 , 5281-5289, (2010)] |
|
Equilibrium vs ground-state planarity of the CONH linkage.
2007-04-05 [J. Phys. Chem. A 111(13) , 2574-86, (2007)] |
|
Analysis of Fungicide Sensitivity and Genetic Diversity amon...
2015-06-01 [Plant Pathol. J. 31 , 115-22, (2015)] |