Kinetics of cutinase catalyzed transesterification in AOT reversed micelles: modeling of a batch stirred tank reactor.
C M Carvalho, M R Aires-Barros, J M Cabral
Index: J. Biotechnol. 81(1) , 1-13, (2000)
Full Text: HTML
Abstract
A transesterification process is analyzed in its multiple kinetic components that include the determination of the kinetic constants for both substrates, butyl acetate (BAc) and hexanol (H), involved in the alcoholysis reaction and for the products formed (hexyl acetate (HAc) and butanol (B)), participating into the reverse reaction. The order of magnitude of these constants is discussed in relation with the AOT/isooctane reverse micellar system under study. The values of the equilibrium conversion (X(e)) and constant (K(eq)) were also determined. Diffusional limitations were detected for H concentrations lower than 450 mM and the correspondent effectiveness factors were calculated. Above 450 mM H the reaction is kinetically controlled. The operation of a batch stirred tank reactor (BSTR) was modeled considering the integrated rate equation for reversible kinetics.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
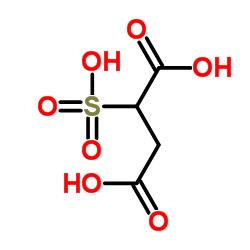 |
2-Sulfosuccinic acid
CAS:5138-18-1 |
C4H6O7S |
|
Physicochemical characterization of drug-loaded rigid and el...
2011-06-30 [Int. J. Pharm. 412(1-2) , 142-7, (2011)] |
|
Permanent enzyme microencapsulation in reverse micellar medi...
1995-03-31 [Ann. N. Y. Acad. Sci. 750 , 89-93, (1995)] |
|
Effect of added alpha-lactalbumin protein on the phase behav...
2003-05-15 [J. Colloid. Interface Sci. 261(2) , 514-23, (2003)] |
|
The effect of pilocarpine on ocular levobunolol absorption f...
1994-01-01 [J. Ocul. Pharmacol. 10(4) , 605-15, (1994)] |
|
Solvent and rotational relaxation of coumarin-153 and coumar...
2013-02-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 102 , 371-8, (2013)] |