Organic & Biomolecular Chemistry
2011-12-07
Enzymatic enantiomeric resolution of phenylethylamines structurally related to amphetamine.
Lourdes Muñoz, Anna M Rodriguez, Gloria Rosell, M Pilar Bosch, Angel Guerrero
Index: Org. Biomol. Chem. 9(23) , 8171-7, (2011)
Full Text: HTML
Abstract
Both enantiomers of several phenylethylamines, structurally related to amphetamine, have been prepared in good yields and excellent enantiomeric purity by enzymatic kinetic resolution using CAL-B and ethyl methoxyacetate as the acyl donor. In the case of the 4-hydroxyderivative of amphetamine (compound 4i), the S enantiomer racemized possibly in a dynamic kinetic resolution (DKR) under the enzymatic conditions used.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
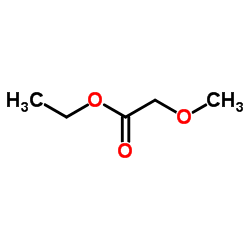 |
Ethyl methoxyacetate
CAS:3938-96-3 |
C5H10O3 |
Related Articles:
More...
|
Molecular basis for the enhanced lipase-catalyzed N-acylatio...
2006-11-01 [ChemBioChem. 7(11) , 1745-9, (2006)] |
|
Hydration structure of poly(2-methoxyethyl acrylate): compar...
2010-01-01 [J. Biomater. Sci. Polym. Ed. 21(14) , 1925-35, (2010)] |
|
Reagents for (ir)reversible enzymatic acylations.
2003-07-21 [Org. Biomol. Chem. 1(14) , 2405-15, (2003)] |
|
Application of automated matrix-assisted laser desorption/io...
2001-01-01 [Rapid Commun. Mass Spectrom. 15(15) , 1327-33, (2001)] |
|
Aneuploidy-inducing chemicals in yeast evaluated by the micr...
1986-05-01 [Mutat. Res. 174(1) , 11-3, (1986)] |