AM 103 Suppliers
Total count: 3Updated Date: 2025-05-19 18:14:17

- China(Mainland)
- Contact: Yang
- Phone: 0086-021-52280163
- Email: sales@echemcloud.com
- Website: http://www.echemcloud.com
- Product Name: AM 103?
- Updated Date: 2024-07-15 12:30:16
- Purity: 99.21%
- More information
Inquiry

- China(Mainland)
- Contact: Tony Lai
- Phone: 13564518121
- Email: order@dcchemicals.com
- Website: http://www.biomedgen.cn
- Product Name: AM103
- Updated Date: 2025-09-03 11:13:17
- Purity: 98.9%
- More information
¥Inquiry
1g

- China(Mainland)
- Contact: Tony Cao
- Phone: 13564518121
- Email: sales@dcchemicals.com
- Website: http://www.dcchemicals.com
- Product Name: AM103
- Updated Date: 2024-07-17 15:20:26
- Purity: 98.0%
- More information
¥800.0
100mg
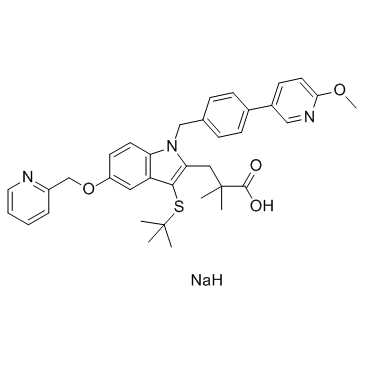
AM 103
- CAS Number: 1147872-22-7
- Molecular Formula: C36H40N3NaO4S
- Molecular Weight: 633.78
Price: $1920/1mg
Reference only. More AM 103 price
Related product suppliers
Check more product suppliers