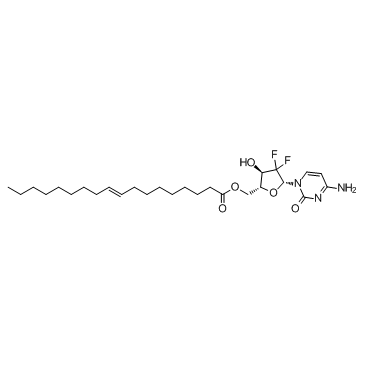
210829-30-4
| Name | [(2R,3R,5R)-5-(4-amino-2-oxopyrimidin-1-yl)-4,4-difluoro-3-hydroxyoxolan-2-yl]methyl (E)-octadec-9-enoate |
|---|---|
| Synonyms |
Gemcitabine elaidate [INN]
2'-Deoxy-2',2'-difluoro-5'-O-[(9E)-9-octadecenoyl]cytidine CO-101 UNII-231C73W7LG Gemcitabine elaidate CP-4126 Gemcitabine elaidate (USAN/INN) Gemcitabine (elaidate) |
| Description | Gemcitabine elaidate(CP-4126; CO-101) is a lipophilic, unsaturated fatty acid ester derivative of gemcitabine (dFdC), an antimetabolite deoxynucleoside analogue, with potential antineoplastic activity.IC50 value:Target: Gemcitabine analogUpon hydrolysis intracellularly by esterases, the prodrug gemcitabine is converted into the active metabolites difluorodeoxycytidine di- and tri-phosphate (dFdCDP and dFdCTP) by deoxycytidine kinase. dFdCDP inhibits ribonucleotide reductase, thereby decreasing the deoxynucleotide pool available for DNA synthesis; dFdCTP is incorporated into DNA, resulting in DNA strand termination and apoptosis. Due to its lipophilicity, gemcitabine 5'-elaidic acid ester exhibits an increased cellular uptake and accumulation, resulting in an increased conversion to active metabolites, compared to gemcitabine. In addition, this formulation of gemcitabine may be less susceptible to deamination and deactivation by deoxycytidine deaminase. Check for active clinical trials or closed clinical trials using this agent. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 631.4±65.0 °C at 760 mmHg |
| Molecular Formula | C27H43F2N3O5 |
| Molecular Weight | 527.644 |
| Flash Point | 335.7±34.3 °C |
| Exact Mass | 527.317078 |
| PSA | 117.66000 |
| LogP | 7.70 |
| Vapour Pressure | 0.0±4.2 mmHg at 25°C |
| Index of Refraction | 1.536 |
| Storage condition | 2-8℃ |