1202055-34-2
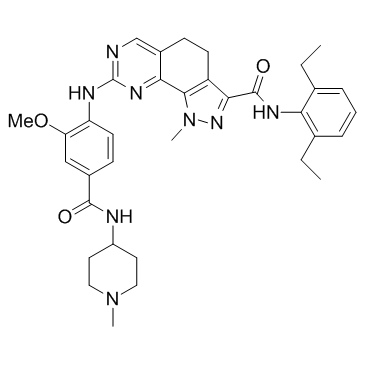
| Name | N-(2,6-diethylphenyl)-8-({2-methoxy-4-[(1-methylpiperidin-4-yl)carbamoyl]phenyl}amino)-1-methyl-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide |
|---|---|
| Synonyms |
NMS P715 analog
NMS-P715 analog N-(2,6-Diethylphenyl)-8-({2-methoxy-4-[(1-methyl-4-piperidinyl)carbamoyl]phenyl}amino)-1-methyl-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide NMS-P 715 analog NMS-P715 NMS-P715 (analog) |
| Description | NMS-P715 analog is an inhibitor of MPS1, with an IC50 of 84 nM. |
|---|---|
| Related Catalog | |
| Target |
MPS1:84 nM (IC50) PLK1:237 nM (IC50) Aur-A:1450 nM (IC50) |
| In Vitro | NMS-P715 analog (Compound 14) is an inhibitor of MPS1, with an IC50 of 84 nM; also less active on Aur-A, CDK2/A and PLK1 (IC50, 1.45, >10, 0.237 μM). In addition, NMS-P715 analog shows inhibitory effect on human tumor cell line (A2780) with an IC50 of 150 nM. |
| Kinase Assay | The potency of the compounds (NMS-P715 analog, etc.) towards MPS1, Aur-A, CDK2/A, and PLK1 is determined using either a strong anion exchanger based assay or P81 Multiscreen plate, both based on the specific measurement of radioactive phospho-transfer to the substrate. For each enzyme, the absolute Km values for ATP and the specific substrate are initially determined and each assay is then run at optimized [ATP] (2•αKm) and [substrate] (5•Km) concentrations. These conditions enabled direct comparison of IC50 values across the different kinases to evaluate the selectivity profile. Activity is measured using 5 nM of MPS1 recombinant protein in 50 mM HEPES pH 7.5, 2.5 mM MgCl2, 1 mM MnCl2, 1 mM DTT, 3 μM NaVO3, 2 mM β-glycerophosphate, 0.2 mg/mL BSA, 200 μM P38-βtide substrate-peptide (KRQADEEMTGYVATRWYRAE) and 8 μM ATP with 1.5 nM 33P-γ-ATP[1]. |
| Cell Assay | A2780 ovarian carcinoma cells (ECACC) cultured in RPMI medium, supplemented with 10% fetal calf serum (FCS) and 2 mM L-Glutamine are seeded in 384 well-plates and treated with compounds (NMS-P715 analog, etc.) dissolved in 0.1% DMSO 24 hours after seeding. The cells are incubated at 37°C and 5% CO2 and after 72 hours the plates are processed using CellTiter-Glo assay. Inhibitory activity is evaluated comparing treated versus control data using Assay Explorer software. IC50 of proliferation is calculated using sigmoidal interpolation curve fitting[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C35H42N8O3 |
| Molecular Weight | 622.760 |
| Exact Mass | 622.338013 |
| PSA | 126.30000 |
| LogP | 3.69 |
| Index of Refraction | 1.676 |