173937-91-2
| Name | atrasentan |
|---|---|
| Synonyms |
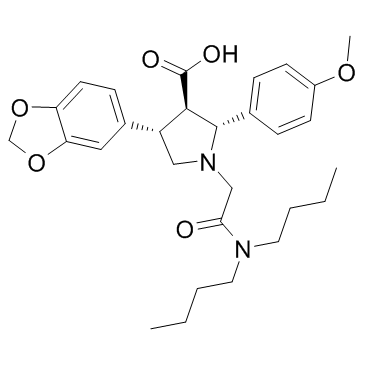
[2R,3R,4S]-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-[[(N,N-dibutylamino)-carboxyl]methyl]pyrrolidine-3-carboxylic acid
(2R,3R,4S)-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(((N,N-dibutylamino)carbonyl)methyl)pyrrolidine-3-carboxylic acid (2R,3R,4S)-4-(1,3-Benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic acid (2R,3R,4S)-(+)-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(N,N-di(n-butyl)aminocarbonylmethyl)-pyrrolidine-3-carboxylic acid |
| Description | Atrasentan is an endothelin receptor antagonist with IC50 of 0.0551 nM for ETA. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.055 nM (ETA) |
| In Vitro | Atrasentan (ABT-627, 0-50 μM) significantly inhibits LNCaP and C4-2b prostate cancer cell growth. ABT-627 in conbination with Taxotere elicits a significantly greater loss of viable prostate cancer cells relative to either agent alone and shows greater degree of down-regulation of the NF-κB DNA binding activity[2]. Atrasentan profoundly induces several CYPs and drug transporters (e.g. 12-fold induction of CYP3A4 at 50 μM). It is a moderate P-gp inhibitor (IC50 in P388/dx cells=15.1±1.6 μM) and a weak BCRP inhibitor (IC50 in MDCKII-BCRP cells=59.8±11 μM)[3]. |
| In Vivo | Atrasentan (3 mg/kg, p.o.) inhibits the pressor response induced by big endothelin-1 (1 nmol/kg) in pithed rats[1]. Aatrasentan (ABT-627, 10 mg/kg, i.p.) as well as Taxotere alone inhibited the C4-2b tumor growth within the bone environment to some extent in the SCID-hu model[2]. |
| Kinase Assay | Cells are incubated and treated with Atrasentan. They are then washed twice with PBS and lysed in ice-cold lysis buffer [20 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium PPi, 1 mM β-glycerophosphate, 1 mM sodium orthovanadate, 1 μg/mL leupeptin, and 1 mM PMSF]. The extracts are centrifuged to remove cellular debris, and the protein content of the supernatants is determined using the bicinchoninic acid (BCA) protein assay reagent. Proteins (150 μg) are incubated with gentle rocking at 4°C overnight with immobilized Akt antibody cross-linked to agarose hydrazide beads. After the Akt is selectively immunoprecipitated from the cell lysates, the immunoprecipitated products are washed twice with lysis buffer and twice with kinase assay buffer [25 mM Tris (pH 7.5), 10 mM MgCl2, 5 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 2 mM DTT] and then resuspended in 40 μL of kinase assay buffer containing 200 μM ATP and 1 μg GSK-3α/β fusion protein. The kinase assay reaction is allowed to proceed at 30°C for 30 min and stopped by the addition of Lamelli SDS sample buffer. Reaction products are resolved by 10% SDS-PAGE, followed by Western blotting with antiphosphorylated GSK-3α/β antibody. For analysis of the total amount of Akt, 40 μg of protein from the lysate samples are resolved by 10% SDS-PAGE, followed by Western blotting with anti-Akt antibody. |
| Cell Assay | All three prostate cancer cell lines (LNCaP, C4-2b, and PC-3 cells) are seeded at a density of 3 × 103 cells per well in 96-well microtiter culture plates. After overnight incubation, the medium is removed and replaced with a fresh medium containing different concentrations of ABT-627 (0-50 μM) diluted from a 10-mM stock. After 72 h of incubation with drug, 20 μL of MTT solution (5 mg/mL in PBS) are added to each well and incubated further for 2 h. Upon termination, the supernatant is aspirated and the MTT formazan formed by metabolically viable cells is dissolved in isopropanol (100 μL). The plates are mixed for 30 min on a gyratory shaker, and the absorbance is measured at 595 nm on a plate reader. |
| Animal Admin | YM598 (0.3, 1, and 3 mg/kg), atrasentan (0.3, 1, and 3 mg/kg), or 0.5% methyl cellulose as vehicle is orally administered to rats with a dosing cannula. Dosing volume of the test substances and vehicle is set at 5 mL/kg. Approximately 20 min after administration of compounds, the rats are anesthetized with sodium pentobarbital, and then pithed and ventilated 30 min after dosing. Approximately 1 h after oral administration of compounds, big endothelin-1 (1 nmol/kg) is intravenously administered, and blood pressure is measured. In these two experiments, the dose of test compound that cause 50% inhibition (ID50) of the big endothelin-1-induced increase in diastolic blood pressure is determined by linear regression analysis. |
| References |
| Density | 1.188g/cm3 |
|---|---|
| Boiling Point | 659.4ºC at 760mmHg |
| Molecular Formula | C29H38N2O6 |
| Molecular Weight | 510.62200 |
| Flash Point | 352.6ºC |
| Exact Mass | 510.27300 |
| PSA | 88.54000 |
| LogP | 4.63190 |
| Vapour Pressure | 2.76E-18mmHg at 25°C |
| Storage condition | 2-8℃ |
| Precursor 7 | |
|---|---|
| DownStream 0 | |